Scientists discover how to 'switch off' autoimmune diseases
Scientists have made an important breakthrough in the fight against debilitating autoimmune diseases such as multiple sclerosis by revealing how to stop cells attacking healthy body tissue.
Rather than the body's immune system destroying its own tissue by mistake, researchers at the University of Bristol have discovered how cells convert from being aggressive to actually protecting against disease.
The study, funded by the Wellcome Trust, is published today [03 September] in Nature Communications.
It's hoped this latest insight will lead to the widespread use of antigen-specific immunotherapy as a treatment for many autoimmune disorders, including multiple sclerosis (MS), type 1 diabetes, Graves' disease and systemic lupus erythematosus (SLE).
MS alone affects around 100,000 people in the UK and 2.5 million people worldwide.
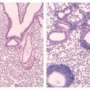
Scientists were able to selectively target the cells that cause autoimmune disease by dampening down their aggression against the body's own tissues while converting them into cells capable of protecting against disease.
This type of conversion has been previously applied to allergies, known as 'allergic desensitisation', but its application to autoimmune diseases has only been appreciated recently.
The Bristol group has now revealed how the administration of fragments of the proteins that are normally the target for attack leads to correction of the autoimmune response.
Most importantly, their work reveals that effective treatment is achieved by gradually increasing the dose of antigenic fragment injected.
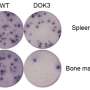
In order to figure out how this type of immunotherapy works, the scientists delved inside the immune cells themselves to see which genes and proteins were turned on or off by the treatment.
They found changes in gene expression that help explain how effective treatment leads to conversion of aggressor into protector cells. The outcome is to reinstate self-tolerance whereby an individual's immune system ignores its own tissues while remaining fully armed to protect against infection.
By specifically targeting the cells at fault, this immunotherapeutic approach avoids the need for the immune suppressive drugs associated with unacceptable side effects such as infections, development of tumours and disruption of natural regulatory mechanisms.
Professor David Wraith, who led the research, said: "Insight into the molecular basis of antigen-specific immunotherapy opens up exciting new opportunities to enhance the selectivity of the approach while providing valuable markers with which to measure effective treatment. These findings have important implications for the many patients suffering from autoimmune conditions that are currently difficult to treat."
This treatment approach, which could improve the lives of millions of people worldwide, is currently undergoing clinical development through biotechnology company Apitope, a spin-out from the University of Bristol.
More information: 'Sequential transcriptional changes dictate safe and effective antigen-specific immunotherapy' by Bronwen R. Burton, Graham J. Britton, Hai Fang, Johan Verhagen, Ben Smithers Catherine A. Sabatos-Peyton, Laura J. Carney, Julian Gough, Stephan Strobel and David C. Wraith in Nature Communications.