A scalable platform for growing heart muscle cells may lead to repair of damaged heart cells
The ability to grow human heart muscle cells in bulk could help routine replacement of heart cells damaged during a heart attack and may also improve testing of pharmaceutical drugs on heart cells, shows A*STAR research1.
Cardiovascular diseases such as heart attacks and strokes are the world's leading causes of death. During a heart attack some of the heart muscle cells become starved of blood and die. These cells are rarely replaced due to the heart's limited regenerative ability, which means that patients have to live with the limitations of a damaged heart.
Steve Oh led a team of researchers from the A*STAR Bioprocessing Technology Institute in designing a prototype 'platform' that enables heart muscle cells, or cardiomyocytes, to be produced from human pluripotent stem cells. Oh believes that this platform could be scaled up to industrial production levels2.
"The key is to be able to produce these cells in bulk," says Oh. "Eventually we will be able to produce trillions of cells, but our current work involves developing the prototype process that is generating hundreds of millions of cells per batch."
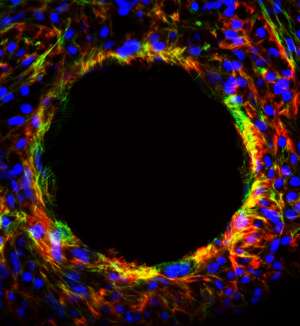
Traditionally, tissue culture is grown on a flat plate, but Oh's team used 'microcarriers'—tiny polystyrene spheres that are electrically charged and coated with a protein—as the base for their culture (see image).
"Stem cells like to grow as clusters," says Oh. "These microcarriers enable them to do that while suspended in a spinner flask, which is better for bulk production," says Oh.
Researchers typically agitate stem cell cultures to help the cells receive nutrients and prevent them from clumping. Oh's team conducted extensive research into the optimal levels of agitation required to increase the number of cells produced. They showed that too much agitation in the first few days of culture growth is detrimental3.
The team developed two effective protocols: one using stirring and another employing a rocking motion. "Both approaches have potential for industrial scale production," says Oh.
They also created an automated video analysis technique to efficiently quantify the cardiomyocytes produced using their process4. The technique was used to visualize the newly grown heart cells as they regularly contract or beat—just as cardiomyocytes do in a living heart (see video).
"Before the video analysis system, we had to depend on experienced eyes to identify beating microcarrier–cell aggregates," says Oh.
The next step for the group is to scale up production to more commercially useful levels. The process currently uses a 50 milliliter flask of microcarriers and stem cells, but, says Oh, "We need to take it to a one liter controlled bioreactor."
More information: 1. Chen, A., Ting, S., Seow, J., Reuveny, S. & Oh, S. Considerations in designing systems for large scale production of human cardiomyocytes from pluripotent stem cells. Stem Cell Research & Therapy 5, 12 (2014). dx.doi.org/10.1186/scrt401
2. Lam, A. T.-L., Chen, A. K.-L., Li, J., Birch, W. R., Reuveny, S. & Oh, S. K.-W. Conjoint propagation and differentiation of human embryonic stem cells to cardiomyocytes in a defined microcarrier spinner culture. Stem Cell Research & Therapy 5, 110 (2014). dx.doi.org/10.1186/scrt498
3. Ting, S., Chen, A., Reuveny, S. & Oh, S. An intermittent rocking platform for integrated expansion and differentiation of human pluripotent stem cells to cardiomyocytes in suspended microcarrier cultures. Stem Cell Research 13, 202–213 (2014). dx.doi.org/10.1016/j.scr.2014.06.002
4. Ting, S., Liew, S. J., Japson, F., Shang, F., Chong, W. K. et al. Time-resolved video analysis and management system for monitoring cardiomyocyte differentiation processes and toxicology assays. Biotechnology Journal 9, 675–683 (2014). dx.doi.org/10.1002/biot.201300262















