Changes in a blood-based molecular pathway identified in Alzheimer's disease
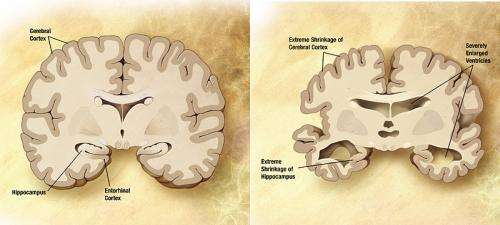
By the time most people receive a diagnosis of Alzheimer's disease—based on clinical signs of mental decline—their brains have already suffered a decade or more of damage. But although the mechanisms that spur the destruction of neurons in Alzheimer's disease are not yet fully understood, two well-documented signs of the condition are accumulation of the amyloid-β peptide (the main component of plaques found in Alzheimer's patient brains) and chronic inflammation. New research from Rockefeller University, published March 16 in the Proceedings of the National Academy of Sciences, identifies a bridge between the two. That bridge, a molecular cascade known as the contact system, may provide opportunities for early diagnosis of the disease through simple blood tests.
"People have been looking for a long time for markers for Alzheimer's disease," says Sidney Strickland, head of the Patricia and John Rosenwald Laboratory of Neurobiology and Genetics. But current diagnostic tests for pre-symptomatic Alzheimer's leave much to be desired. Evaluating the level of amyloid-β in the cerebral spinal fluid, for instance, requires an invasive spinal tap procedure.
"Finding a blood biomarker that would let us know through a simple test whether someone is on their way to developing the disease would be a significant advance," says first author Daria Zamolodchikov, a postdoctoral associate in the Strickland lab.
The new study grew from the lab's ongoing work that looks at how the vascular system is involved in Alzheimer's disease. It has been shown that amyloid-β can activate a protein in plasma called factor XII, the first step in a pathway known as the contact system. When activated, this system leads to the release of a small peptide called bradykinin, a molecule known to promote potentially damaging inflammation. Although some studies have found these molecules in the cerebral spinal fluid and brain tissue of Alzheimer's patients, no one had studied them in Alzheimer's patient plasma.
Using plasma from people with and without diagnosed Alzheimer's disease, the researchers measured the activation levels of the contact system. They found increased activation of this system in the plasma of Alzheimer's patients, potentially implicating it in the inflammatory pathology of the disease. Moreover, in a subset of patients whose amyloid-β levels in the cerebral spinal fluid were known, the researchers demonstrated a positive correlation between activation of the contact system and changes in cerebral spinal fluid amyloid-β levels, which as mentioned above are correlated with the development of Alzheimer's.
The researchers found similar activation of the contact system in mouse models of Alzheimer's, which are genetically modified to overproduce amyloid-β. They then conducted a follow-up experiment with healthy mice. "We went one step further and took completely normal wild-type mice and injected them with amyloid-β. We found that on its own, injection with amyloid-β can activate this system. It's a proof of principle in a complex environment," says Zamolodchikov.
These findings will need to be supported by studies in larger patient populations and longitudinal studies, but they could eventually open the door to diagnosis of pre-symptomatic Alzheimer's based on blood levels of these molecules.
The contact system may also offer a new approach to therapies for Alzheimer's disease, since inhibition of the pathway could blunt some of the inflammatory aspects of the disease. One concern is that the contact system is also involved in blood clotting and inhibition might carry a risk of bleeding. However, people with a defect in this system do not have hemophilia. Thus, inhibition of this pathway might slow progression of the disease without increasing the risk of hemorrhage.
More information: "Activation of the factor XII-driven contact system in Alzheimer's disease patient and mouse model plasma." PNAS 2015 ; published ahead of print March 16, 2015, DOI: 10.1073/pnas.1423764112