August 17, 2015 report
Researchers find intestinal symbionts induce distinct T-regulator cells
(Medical Xpress)—The body's subpopulation of T-regulatory (Treg) cells modulates the immune system, helps the body to recognize and tolerate self-antigens, and is believed to be responsible for the abrogation of autoimmune disease. Recently, immunology researchers have reported in the journal Science that symbionts of the gut microbiota induce a distinct population of T-regulatory cells that express the transcription factor Foxp3.
This Treg population constrains immuno-inflammatory responses in the gut, and is induced by a specific array of bacterial species. Unexpectedly, this induction requires a transcription factor called RORγ, which has previously been known to antagonize the Foxp3 transcription factor in other contexts.
The authors point out that Treg cells in various kinds of tissue serve varying purposes beyond immuno-regulatory functions; those found in visceral adipose fat tissue, for instance, regulate metabolic parameters. Others channel inflammatory and regenerative events in injured muscle tissue. The Treg cells reported by the authors are found in the lamina propria tissues of the colon, where they modulate responses to commensal microbes—those that benefit from residence in another organism, which neither help nor harm it.
In germ-free mice with knocked-out gut microbiota, researchers have noted a correspondingly decreased abundance of Treg cells, evidence that the expression of the regulators is driven by the microbiota.
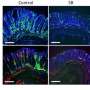
The researchers searched for tissue-specific adaptations in the Treg cells by looking at hundreds of transcription factors that were specific to those in the colon. "To our surprise," the authors write, "the most differential of these TFs proved to be Rorc (encodes RORγ). RORγ controls many aspects of immunocyte differentiation but is perhaps best known... as a reciprocal antagonist of FoxP3 during in vitro differention..."
Further, this expression of the RORγ transcription factor appears in mice between 15 and 25 days old, coincident with the changes in gut microbiota that occur during normal maturation, when mice transition to solid food. And evidence for this microbiotic induction of Treg cells continued to accumulate in the study: Individual antibiotics had little effect on the expression of Treg cells, but broad-spectrum antibiotics strongly affected their population, indicating that the contribution of several different microbes was responsible for inducing the expression of RORγ Treg cells.
Identifying RORγ in rodents, the researchers sought to discover whether it was also expressed in human intestinal Treg cells. They stained both healthy and inflamed cells from Crohn's biopsies and did, indeed, find the RORγ transcription factor in both contexts.
In a model of colitis in mice, the researchers found that colonic Treg cells lacking RORγ resulted in an exacerbation of inflammatory severity. Thus, they believe that RORγ has a specific role in the maintenance of colonic homeostasis.
"Thus, RORγ contributes unexpectedly but in an important way to the Treg response to commensal microbes. This role contrasts with the accepted dichotomy between FoxP3 and RORγ, a notion stemming mainly from their antagonism in vitro; perhaps this relation has been overinterpreted," the authors write. They also speculate that human genetic variants associated with inflammatory bowel disease might involve balancing effects in both effector and regulatory T cells.
More information: "Individual intestinal symbionts induce a distinct population of RORγ+ regulatory T cells." Science DOI: 10.1126/science.aaa9420
ABSTRACT
T regulatory cells that express the transcription factor Foxp3 (Foxp3+ Treg) promote tissue homeostasis in several settings. We now report that symbiotic members of the human gut microbiota induce a distinct Treg population in the mouse colon, which constrains immuno-inflammatory responses. This induction—which we find to map to a broad, but specific, array of individual bacterial species—requires the transcription factor Rorγ, paradoxically, in that Rorγ is thought to antagonize FoxP3 and to promote T helper 17 (TH17) cell differentiation. Rorγ's transcriptional footprint differs in colonic Tregs and TH17 cells and controls important effector molecules. Rorγ, and the Tregs that express it, contribute substantially to regulating colonic TH1/TH17 inflammation. Thus, the marked context-specificity of Rorγ results in very different outcomes even in closely related cell-types.
© 2015 Medical Xpress