Clinical trial of chikungunya vaccine opens
An experimental vaccine to protect against the mosquito-borne illness chikungunya is being tested in a Phase 2 trial sponsored by the National Institutes of Health. Results from an initial trial of the vaccine, which was developed by scientists at the NIH National Institute of Allergy and Infectious Diseases (NIAID), were reported in 2014. In that study, all 25 vaccine recipients developed robust immune responses and no safety concerns were noted. The new trial is designed to enroll 400 healthy adult volunteers aged 18 to 60 years old at six sites in the Caribbean. It will continue to gather data on the candidate vaccine's safety and ability to elicit immune responses, including antibodies.
The hallmark symptoms of chikungunya are severe joint pain accompanied by fever and headache. The pain typically eases after about a week but can persist for months or years in some cases. There are no specific treatments for chikungunya infection and no vaccine to prevent it.
Since its appearance in the Western Hemisphere in late 2013, cases of chikungunya have skyrocketed. So far in 2015, more than 621,000 suspected and confirmed cases have been reported throughout the Americas.
"The recent re-emergence of chikungunya virus in this hemisphere has rapidly become a significant health burden," said NIAID Director Anthony S. Fauci, M.D. "Our chikungunya vaccine development efforts are part of a broader research effort to prevent, diagnose, treat and ultimately control this painful illness, which can strike anyone unlucky enough to be bitten by an infected mosquito."
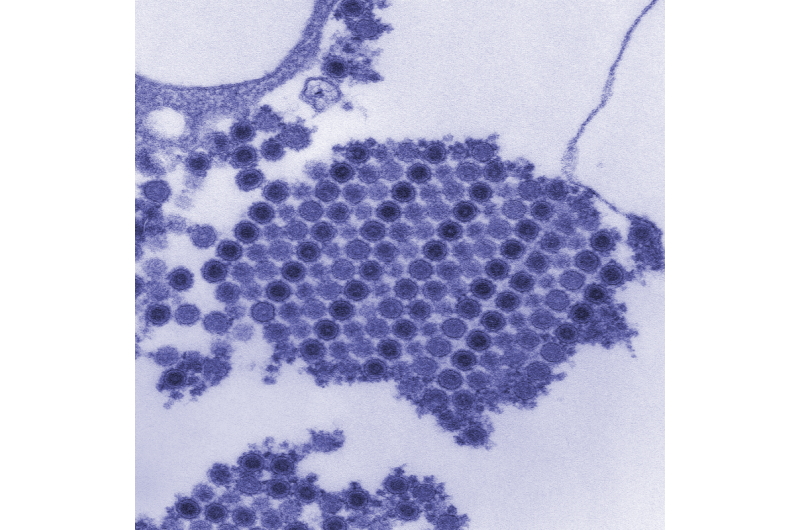
The experimental vaccine, developed by investigators at NIAID's Vaccine Research Center, uses virus-like particles (VLPs) instead of either inactivated or weakened whole virus. VLP vaccines can stimulate immune responses comparable to those resulting from naturally acquired immunity following infection and, because virus is not needed to produce VLP vaccines, they do not need to be prepared in high-level biocontainment facilities.
Eligible volunteers will be randomly assigned to enroll into one of two groups of 200 people each. Study participants will receive either two doses of the candidate vaccine spaced 28 days apart or two doses of an inactive placebo. Blood samples will be drawn at multiple time points following the injections to assess whether the candidate vaccine prompted the production of antibodies to chikungunya virus.
More information: Lee-Jah Chang et al. Safety and tolerability of chikungunya virus-like particle vaccine in healthy adults: a phase 1 dose-escalation trial, The Lancet (2014). DOI: 10.1016/S0140-6736(14)61185-5