Could a gene-editing tweak make pigs organ donors for ailing humans?
Despite their slovenly habits in agricultural settings, pigs raised in biomedical labs are clean enough that many humans would welcome - indeed, do welcome - the use of their tissue for life-saving transplants. Transplanted heart valves routinely come from pigs as well as cows.
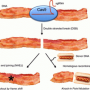
But the dream of transplanting whole pig organs into humans who need new hearts, livers, kidneys or lungs - xenotransplantation - is not so simple a matter. In addition to the usual challenges posed by the immune system's inclination to reject foreign tissue, the use of pig organs to fill the yawning gap between the supply of human organs and demand for them must contend with the problem of PERVs.
PERVs - porcine endogenous retroviruses - are creepy, all right. Under stress, pigs' cells pump out PERVs, which then could infect the human a transplanted organ is meant to save. In a brave new world of xenotransplantation - a world in which pigs could supply organs for some of the 120,000 U.S. patients on the waiting list for a transplantable organ - scientists must find a way to neutralize the threat from PERVs.
And here's where a bit of unexpected help could come from a new gene-editing technique - the CRISPR/Cas9 system, which has made faster, more efficient and more precise the task of paring, replacing and improving problematic genes from DNA.
On Wednesday, this advance was described in the New England Journal of Medicine as a "feat of genetic engineering" that may help to clear the path for xenotransplantation. For NEJM subscribers who may have missed the original article last October in Science, Scripps Research Institute's Dr. Daniel R. Salomon describes how researchers at Harvard "genetically engineered a one-step inactivation of more than 60 copies of PERV, thereby reducing the infectious risk from PERVs by three orders of magnitude."
The strategic snipping of a pig's genome appeared to address both of the key challenges to using pigs as a source of transplantable organs, wrote Salomon: By largely turning off the production of PERVs, researchers have, in principle, reduced both the risk of a recipient's infection with this retrovirus and the likelihood that a recipient's immune system would mount a massive defense against the foreign organ provoked by the presence of these "xenoantigens."
Pigs are hardly close to providing the solution to the world's massive shortage of donor organs. In a lab at the University of Maryland Medical School, surgeons have transplanted more than 50 porcine organs into primates such as baboons in a bid to better understand the immune response in xenotransplantation and the prospects of gene-editing to improve survival. So far, none of the recipients has survived more than a few days.
And Dr. Salomon cautions there are "major obstacles" to the use of the CRISPR/Cas9 gene-editing technique in actual patient care. Scientists still must find ways to ensure against "off-target" effects, such as the disruption of genes that are activated up- or downstream of the manipulated gene sequence. They will need to find ways to make gene-editing a still more efficient process, he wrote. And the human body's immune response to a foreign organ needs to be understood in far better detail if it is to be thwarted, he added.
"Nonetheless, it is important to acknowledge that knocking out 60 different copies of PERV polymerase genes in a single cell will lead to the production of a new pig strain for xenotransplantation, one that has a dramatically decreased risk of transmitting PERVs to immunosuppressed human patients," he wrote.
©2016 Los Angeles Times
Distributed by Tribune Content Agency, LLC.