Three candidate Zika vaccines protect rhesus monkeys against the disease
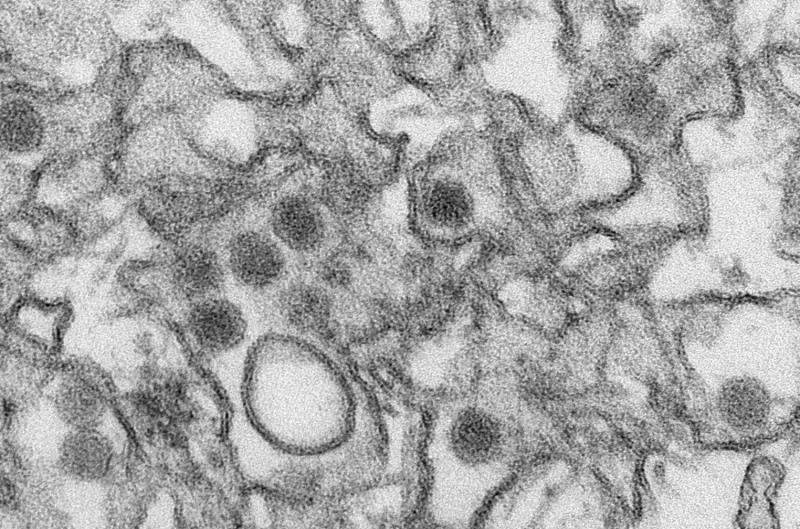
The September 9 issue of Science features a cover article by Brazilian and U.S. researchers with the results of trials showing that three different candidate Zika vaccines protected rhesus monkeys against the disease. The same issue of the journal includes an editorial about the vaccine study by renowned U.S. public health expert Michael T. Osterholm, a professor at the University of Minnesota and director of the Center for Infectious Disease Research and Policy at the same university (CIDRAP).
And the journal's Perspective—Infectious Disease section has a commentary by Marc Lipsitch, professor of epidemiology at the Harvard T.H. Chan School of Public Health, Boston (USA), and Benjamin J. Cowling, head of epidemiology and biostatistics at the University of Hong Kong (China).
The co-authors are Paolo Zanotto, coordinator of the Zika Virus Research Network (Rede Zika) in São Paulo, Jean Pierre Peron, a researcher at the University of São Paulo's Biomedical Science Institute (ICB-USP), and Brazilian immunologist Rafael Larocca, a researcher at Harvard Medical School's Center for Virology & Vaccine Research (CVVR) in the US. Larocca is a member of Dan Barouch's team at CVVR and, alongside colleague Peter Abbink, one of the lead authors of this study as well as a previous one by the same group published in Nature.
In the latest study, the researchers tested three vaccines in rhesus monkeys: an inactivated virus formulation, a candidate DNA vaccine, and a third possibility based on a recombinant adenovirus vector expressing the Zika pre-membrane and envelope proteins. All three were effective at warding off infection by the Zika virus strain circulating in Brazil and Puerto Rico.
In another experiment also described in the Science article, the researchers extracted antibodies against Zika from the blood of vaccinated monkeys and injected them into mice that had never had contact with the virus. The antibodies protected the mice against infection, suggesting it might be possible to develop passive immunization strategies similarly to the method used to protect the fetus in a mother infected by cytomegalovirus.
According to Larocca, of the three formulations used in preclinical trials, the only one that will certainly be tested in humans is the platform based on a killed Zika virus developed by the Walter Reed Army Institute of Research in Maryland (USA). "The clinical trial centers are enrolling volunteers and plan to begin tests with them in October".
In his Science editorial, Osterholm expresses the fear that "the road to a Zika vaccine may be long and bumpy." Despite optimistic predictions, in his view it will be "an immense challenge" to demonstrate safety.
"It will likely be necessary to conduct studies involving many thousands of participants to determine if Guillain-Barré syndrome (GBS), a serious autoimmune condition caused by natural Zika virus, is also related to vaccine candidates," Osterholm writes.
Zanotto disagrees, however, saying that data presented by the Oswaldo Cruz Foundation (FIOCRUZ) suggest cases of Guillain-Barré syndrome associated with Zika are not autoimmune because they manifest while the viral infection is still active rather than weeks later, when the organism has acquired immunity against Zika virus.
"In the case of Zika virus, what must be happening is the death of peripheral nerve cells caused directly by acute viral infection," Zanotto said. "There isn't time for an immune response. So in this case, the vaccine could minimize and prevent Guillain-Barré instead of intensifying it. But this needs to be thoroughly corroborated."
In his editorial, Osterholm also writes that "recent follow-up of a vaccine efficacy study for dengue, Zika's cousin flavivirus, suggests a diminishing vaccine-induced antibody response over time." According to him, this means a vaccinated person could be infected by a new dengue strain, or even another flavivirus such as Zika or yellow fever, and suffer the effects of "antibody-dependent enhancement disease," when non-neutralizing antibodies facilitate virus entry into host cells.
Osterholm stresses that the need for a safe and effective Zika vaccine is immediate. "The emergence of Zika in the Americas is a stark reminder of how quickly public health challenges of infectious diseases can change," he writes.
He adds, "Even if such a vaccine is not yet licensed, having it ready for immediate large trials when a regional crisis occurs will be a major advantage over our current reactive system."
The essay by Lipsitch and Cowling also highlights the need for the scientific community to be permanently prepared to deal with emerging infectious diseases. "Repeatedly, emerging infections have caught us by surprise, with no vaccine candidate or only very early-stage candidates available," they write.
They add in a later paragraph, "A key principle of preparedness is to do as much work as possible before an emergency happens, so that the response can be decisive and efficient."
For Zanotto, the scientific community's capacity to respond to epidemic threats has intensified since the outbreak of Ebola in 2014-15.
"Completing proof of concept in less than six months following the outbreak of Zika in Brazil is an unprecedented feat," he said. "Several groups are developing Zika vaccines and a number of platforms are being worked on. A consensus is forming among experts that we need a more proactive approach to potential emerging viral diseases."
The challenge, he added, is the existence of a large number of potentially dangerous pathogens that need to be monitored with care. "We're keeping a close eye on several coronaviruses, such as those that cause Middle East respiratory syndrome, or MERS, and severe acute respiratory syndrome, or SARS, as well as a number of arboviruses that are leaving Africa for Europe, such as Usutu and a second strain of West Nile virus," he said.
More information: M. T. Osterholm. Ebola and Zika: Cautionary tales, Science (2016). DOI: 10.1126/science.aai9078
P. Abbink et al. Protective efficacy of multiple vaccine platforms against Zika virus challenge in rhesus monkeys, Science (2016). DOI: 10.1126/science.aah6157