New evidence for genetic basis of autism found
Scientists at Cold Spring Harbor Laboratory (CSHL) have discovered that one of the most common genetic alterations in autism -- deletion of a 27-gene cluster on chromosome 16 -- causes autism-like features. By generating mouse models of autism using a technique known as chromosome engineering, CSHL Professor Alea Mills and colleagues provide the first functional evidence that inheriting fewer copies of these genes leads to features resembling those used to diagnose children with autism. The study appears in the Proceedings of the National Academy of Sciences in the early online edition during the week of October 3.
"Children normally inherit one copy of a gene from each parent. We had the tools to see whether copy number changes found in kids with autism were causing the syndrome," explains Mills. In 2007, Professor Michael Wigler, also at CSHL, revealed that some children with autism have a small deletion on chromosome 16, affecting 27 genes in a region of our genomes referred to as 16p11.2. The deletion -- which causes children to inherit only a single copy of the 27-gene cluster -- is one of the most common copy number variations (CNVs) associated with autism.
"The idea that this deletion might be causing autism was exciting," says Mills. "So we asked whether clipping out the same set of genes in mice would have any effect."
After engineering mice that had a chromosome defect corresponding to the human 16p11.2 deletion found in autism, Mills and her team analyzed these models for a variety of behaviors, as the clinical features of autism often vary widely from patient to patient, even within the same family.
"Mice with the deletion acted completely different from normal mice," explains Guy Horev, a Postdoctoral Fellow in the Mills laboratory and first author of the study. These mice had a number of behaviors characteristic of autism: hyperactivity, difficulty adapting to a new environment, sleeping deficits, and restricted, repetitive behaviors.
Interestingly, mice that had been engineered to carry an extra copy, or duplication, of the 16p11.2 region did not have these characteristics, but instead, had the reciprocal behaviors. For each behavior, the deletion had a more dire consequence than the duplication, indicating that gene loss was more severe. This might explain why 16p11.2 duplications are detected much more frequently than deletions within the human population, and why patients with 16p11.2 deletions tend to be diagnosed earlier than those with duplications.
The mouse models also revealed a potential link between 16p11.2 deletion and survival, as about half the mice died following birth. Whether these findings extend to the human population might be answered by future studies that investigate the link between this deletion and unexplained cases of infant death.
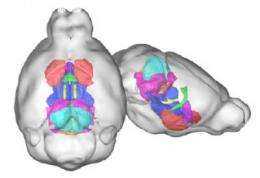
The researchers also used MRI to identify specific regions of the brain that were altered in the autism models, revealing that eight different parts of the brain were affected. The group is now working to identify which gene or group of genes among the 27 that are located within the deleted region is responsible for the behaviors and brain alterations observed.
"Alea Mills has created a valuable resource for everyone engaged in autism research. The technical skill is extraordinary in creating mouse models bearing a human genetic variant that has been associated with autism," says Dr. Gerald Fischbach, Director of Life Sciences and Simons Foundation Autism Research Initiative (SFARI).
These mice will be invaluable for pinpointing the genetic basis of autism and for elucidating how these alterations affect the brain. They could also be used for inventing ways to diagnose children with autism before they develop the full-blown syndrome, as well as for designing clinical interventions.
More information: Guy Horev, et al., "Dosage-dependent phenotypes in models of 16p11.2 lesions found in autism," Proceedings of the National Academy of Sciences, October 3, 2011.













