Brain-imaging technique predicts who will suffer cognitive decline over time
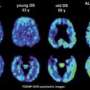
Cognitive loss and brain degeneration currently affect millions of adults, and the number will increase, given the population of aging baby boomers. Today, nearly 20 percent of people age 65 or older suffer from mild cognitive impairment and 10 percent have dementia.
UCLA scientists previously developed a brain-imaging tool to help assess the neurological changes associated with these conditions. The UCLA team now reports in the February issue of the journal Archives of Neurology that the brain-scan technique effectively tracked and predicted cognitive decline over a two-year period.
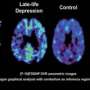
The team has created a chemical marker called FDDNP that binds to both plaque and tangle deposits — the hallmarks of Alzheimer's disease — which can then be viewed using a positron emission tomography (PET) brain scan, providing a "window into the brain." Using this method, researchers are able to pinpoint where in the brain these abnormal protein deposits are accumulating.
"We are finding that this may be a useful neuro-imaging marker that can detect changes early, before symptoms appear, and it may be helpful in tracking changes in the brain over time," said study author Dr. Gary Small, UCLA's Parlow–Solomon Professor on Aging and a professor of psychiatry at the Semel Institute for Neuroscience and Human Behavior at UCLA.
Small noted that FDDNP–PET scanning is the only available brain-imaging technique that can assess tau tangles. Autopsy findings have found that tangles correlate with Alzheimer's disease progression much better than do plaques.
For the study, researchers performed brain scans and cognitive assessments on the subjects at baseline and then again two years later. The study involved 43 volunteer participants, with an average age of 64, who did not have dementia. At the start of the study, approximately half (22) of the participants had normal aging and the other half (21) had mild cognitive impairment, or MCI, a condition that increases a person's risk of developing Alzheimer's disease.
Researchers found that for both groups, increases in FDDNP binding in the frontal, posterior cingulate and global areas of the brain at the two-year follow-up correlated with progression of cognitive decline. These areas of the brain are involved in decision-making, complex reasoning, memory and emotions. Higher initial baseline FDDNP binding in both subject groups was associated with a decline in cognitive functioning in areas such as language and attention at the two-year follow-up.
"We found that increases in FDDNP binding in key brain areas correlated with increases in clinical symptoms over time," said study author Dr. Jorge R. Barrio, who holds UCLA's Plott Chair in Gerentology and is a professor of molecular and medical pharmacology at the David Geffen School of Medicine at UCLA. "Initial binding levels were also predictive of future cognitive decline."
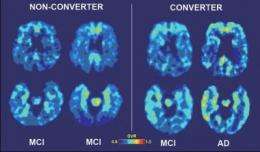
Among the subjects with mild cognitive impairment, the level of initial binding in the frontal and parietal areas of the brain provided the greatest accuracy in identifying those who developed Alzheimer's disease after two years. Of the 21 subjects with MCI, six were diagnosed with Alzheimer's at follow-up, and these six subjects had higher initial frontal and parietal binding values than the other subjects in the MCI group.
In the normal aging subjects, three developed mild cognitive impairment after two years. Two of these three participants had had the highest baseline binding values in the temporal, parietal and frontal brain regions among this group.
Researchers said the next step in research will involve a longer duration of follow-up with larger samples of subjects. In addition, the team is using this brain-imaging technique in clinical trials to help track novel therapeutics for brain aging, such as curcumin, a chemical found in turmeric spice.
"Tracking the effectiveness of such treatments may help accelerate drug discovery efforts," Small, the author of the new book "The Alzheimer's Prevention Program," said. "Because FDDNP appears to predict who will develop dementia, it may be particularly useful in tracking the effectiveness of interventions designed to delay the onset of dementia symptoms and eventually prevent the disease."
Small recently received research approval from the U.S. Food and Drug Administration to use FDDNP–PET to study people with mild cognitive impairment to determine whether a high-potency form of curcumin — a spice with anti-amyloid, anti-tau and anti-inflammatory properties — can prevent Alzheimer's disease and the accumulation of plaques and tangles in the brain.
UCLA owns three U.S. patents on the FDDNP chemical marker. The Office of Intellectual Property at UCLA is actively seeking a commercial partner to bring this promising technology to market.
Small and study authors Jorge R. Barrio and S. C. Huang are among the inventors. Disclosures are listed in the full study.