Can cannabinoid drug used for nausea in chemotherapy relieve sleep apnea?
(Medical Xpress) -- No drug treatments exist to treat sleep apnea, a disorder that affects more than 18 million Americans. Researchers at the University of Illinois at Chicago College of Nursing are working to change that.
A research team led by David Carley, director of the UIC Center for Narcolepsy, Sleep and Health Research, has received a $5 million three-year federal grant to find out if a cannabinoid drug can reduce sleep apnea and protect against diseases linked to it.
The grant, the single largest research award ever received by the UIC College of Nursing, is from the National Heart, Lung and Blood Institute, one of the National Institutes of Health. Known as the Pharmacotherapy of Apnea by Cannabimimetic Enhancement, or PACE trial, it is the first NIH-sponsored multi-site drug trial for obstructive sleep apnea.
“Sleep-related breathing disorders, especially obstructive sleep apnea, pose significant health problems,” says Carley, who is professor of biobehavioral health science at UIC. “Individuals suffering from sleep apnea have an increased risk for coronary heart disease, stroke, high blood pressure and type 2 diabetes.”
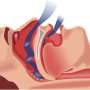
Obstructive sleep apnea is a disorder where breathing is briefly but repeatedly interrupted during sleep. It occurs when the throat muscles fail to keep the airway open during sleep, despite efforts to breathe.
The most common current treatment for sleep apnea is CPAP, or continuous positive airway pressure, in which a machine delivers air through a tightly sealed mask over the nose.
Researchers have attempted to identify drugs to treat sleep apnea for at least 30 years, Carley said.
“By providing a path toward the first viable obstructive sleep apnea drug, our studies could have a tremendous impact on clinical practice,” he said.
One project in his two-part study will be a trial of dronabinol, a cannabinoid drug currently marketed under the trade name Marinol, used to treat nausea and vomiting in chemotherapy patients. It is also prescribed to treat loss of weight and appetite in people who have AIDS.
Patients will be divided into three groups. One group will be given a low dose of the drug, the second group will get a higher dose, and the third, a placebo. Participants will receive the drug once daily before bed for six weeks.
Researchers will measure the drug’s effectiveness by the number of apnea episodes per hour of sleep -- the standard clinical measure of sleep apnea severity. Normal is fewer than five episodes per hour; mild is five to 14; moderate is 15 to 30; and severe is more than 30.
Researchers will also measure blood oxygenation, sleep quality, blood pressure control and daytime alertness, Carley said.
In a second project, UIC researchers will undertake a basic neuroscience study in animal models to define the mechanism of action of dronabinol.
Carley's collaborators are from the UIC departments of pharmacology, medicine, and epidemiology and biostatistics, and from Northwestern University’s neurology department.