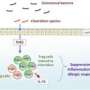
Collaborative effects of multiple bacterial strains in the gut may help prevent onset of certain inflammatory diseases
At first, it may sound alarming to learn that a population of bacteria in your gut is conspiring to suppress your immune system—however, this is actually good news. By identifying the strains responsible, a research team led by Kenya Honda of the RIKEN Center for Integrative Medical Sciences may have uncovered a promising avenue of treatment for certain inflammatory disorders.
Immune cells known as regulatory T (Treg) cells are in part responsible for preventing the immune system from overreacting to foreign molecules or attacking healthy tissue. It is well established that immune function is affected by the diverse microbial community within the digestive tract, and Honda's team previously discovered that bacteria belonging to the genus Clostridium act on this particular immune pathway in mice to exert a strong anti-inflammatory effect.
"We showed that they were responsible for triggering production of Treg cells in the colon of mice," says Honda, "and that oral administration of these strains protected mice against colitis and systemic allergic responses."
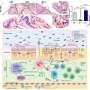
Honda and his colleagues have now verified the existence of an equivalent bacterial population in humans. They obtained a stool sample from a healthy volunteer and subjected it to the same purification regimen that yielded the Clostridia subpopulation identified in mice. When these bacteria were transplanted into the colons of 'germ-free' mice, in which the normal population of gut bacteria is entirely absent, they exerted a potent immunomodulatory effect. Through systematic analysis of this microbial cohort, the researchers zoomed in on a specific subset of 17 distinct Clostridia strains.
These strains collectively secrete a host of signaling molecules that promote Treg cell activation. "None of the organisms alone were nearly as potent as when they were in consortium," says Honda. "This suggests that cooperation between the strains is essential to their therapeutic effects." The collective benefit also appears to pertain to humans; analysis of the gut 'microbiome' in healthy patients versus individuals with ulcerative colitis revealed that all 17 strains were present at significantly lower levels in the latter group.
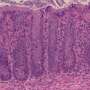
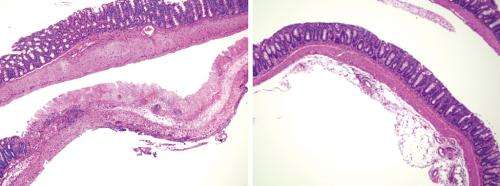
Accordingly, oral administration of this 17-microbe 'cocktail' greatly mitigated intestinal inflammation in mouse models of allergic diarrhea and ulcerative colitis (Fig. 1), suggesting the potential for a more 'natural' treatment of such conditions in humans. "A substantial number of patients don't benefit from existing drugs, which also have considerable adverse effects," says Honda. "We want to clinically test our hypothesis that reconstituting these bacteria to normal levels in patients may help restore immune tolerance and resolve chronic inflammatory processes."
More information: Atarashi, K., et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 500, 232–236 (2013). dx.doi.org/10.1038/nature12331