New study explains why promising dementia drugs failed in clinical trials
Alzheimer's disease is the most common cause of dementia among older people, yet there currently are no effective drugs to stop, slow or prevent disease progression. A study online December 5th in the ISSCR's journal Stem Cell Reports, published by Cell Press, provide interesting clues on why non-steroidal anti-inflammatory drugs (NSAIDs), which have successfully treated molecular signs of Alzheimer's disease in cell and animal models, eventually failed in clinical studies. Whereas the compounds worked in non-neuronal cells lines typically used in pharmaceutical drug screening, the authors found that human neurons are resistant to this class of drugs.
"The results of our study are significant for future drug development approaches, because they imply that compound screening and validation studies might be much more reliable if they are conducted using the human cell type affected by the disease in question," says Oliver Brüstle of the University of Bonn who senior-authored the study together with his colleague Philipp Koch.
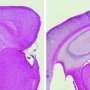
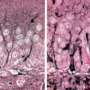
Alzheimer's disease is characterized by the accumulation of compounds called Aβ peptides in the brain, and this process is believed to cause progressive neurodegeneration and dementia. Longer Aβ42 peptides tend to aggregate more than shorter Aβ40 peptides, and a high ratio of Aβ42 to Aβ40 is used as a biomarker of Alzheimer's disease. NSAIDs have been found to modulate Aß processing, resulting in decreased Aß42/40 ratios in several cell and animal models of the disease. But for previously unknown reasons, these drugs failed to delay disease progression in phase 2 and phase 3 clinical trials.
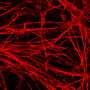
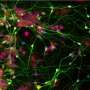
Brüstle and Koch revisited this enigma and for the first time directly tested the effectiveness of NSAIDs in human neurons. They used an induced pluripotent stem cell (iPSC) approach, which involved taking skin cells from patients with Alzheimer's disease, reprogramming these cells into embryonic-like stem cells, and then converting them into neurons. These neurons showed high Aβ42/Aβ40 ratios, which failed to respond to therapeutically relevant concentrations of NSAIDs. In contrast, commonly used non-neuronal cell lines typically employed in drug screening responded strongly, thereby wrongly suggesting efficacy of the drugs.
"The results highlight the importance of testing compounds directly in authentic human cells", says Jerome Mertens, lead author of the study.
"Until recently, it was difficult to obtain native human neurons for drug testing in the field of neurodegenerative diseases. With recent advances in iPSC technology, it has become possible to generate virtually unlimited numbers of human neurons from individual patients," Brüstle says. "We hope that our findings will promote the use of stem cell-derived neurons for drug screening in the field of neurological disorders."
More information: dx.doi.org/10.1016/j.stemcr.2013.10.011