Protein seipin regulates fat development through cytoskeleton remodeling
People with mutations in a gene called BSCL2 suffer from a rare medical condition known as lipodystrophy in which fat tissue is lost from where it is supposed to accumulate while being deposited at unusual sites around the body. However, the way in which these mutations lead to defects in the development and distribution of fat cells, or adipocytes, remained unclear.
Now, Weiping Han and co-workers from the A*STAR Singapore Bioimaging Consortium have dissected the molecular pathway by which seipin, the protein encoded by BSCL2, helps to remodel the cellular scaffolding in fat precursor cells—a process that is essential for proper fat formation.
To reveal the role of seipin in regulating the development of fat cells from precursor cells, Han and his colleagues analyzed the protein players that interact with seipin using mass spectrometry techniques. Seipin is expressed in the endoplasmic reticulum, the organelle involved in trafficking molecules within the cell.
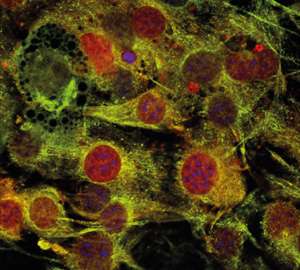
Han and his team discovered that seipin binds to a regulatory protein called 14-3-3β in the cytoplasm. In the presence of the hormone insulin, the 14-3-3β protein, a critical scaffolding molecule in signal transduction, subsequently recruits another protein called cofilin-1, which is otherwise found in the cell nucleus (see image). This second protein modulates actin microfilaments in the cytoskeleton that help to support the internal structure of the cell.
The researchers showed that initiation of this molecular cascade resulted in extensive remodeling of the actin cytoskeleton during fat cell development and maturation. Therefore, blocking the production of any of the four proteins in the pathway—seipin, 14-3-3β, cofilin-1 or actin—led to abnormal fat production in cell culture experiments. "Cytoskeleton remodeling is both necessary for, and a key regulator of, fat formation," explains Han.
Han suggests that the manipulation of any of the four proteins could form the basis of therapies for lipodystrophy. "The targeting of cytoskeleton remodeling may be a potential approach to promoting adipocyte development," he says. "This in turn will alleviate the overloading of lipids in non-adipose tissues and organs and the consequent insulin resistance—hallmarks of lipodystrophy."
Toward that end, Han and his colleagues have engineered a seipin-deficient mouse strain. The model mice develop a disease that looks similar to the lipodystrophy seen in humans. "We will test whether the manipulation of cytoskeleton remodeling can reverse the lipodystrophy phenotype," Han explains, "and also whether this helps to alleviate the other associated metabolic phenotypes, such as insulin resistance."
More information: Yang, W., Thein, S., Wang, X., Bi, X., Ericksen, R. E. et al. BSCL2/seipin regulates adipogenesis through actin cytoskeleton remodelling. Human Molecular Genetics 23, 502–513 (2014). dx.doi.org/10.1093/hmg/ddt444