Cancer virology researchers reveal new pathway that controls how cells make proteins

A serendipitous combination of technology and scientific discovery, coupled with a hunch, allowed University of Pittsburgh Cancer Institute (UPCI) researchers to reveal a previously invisible biological process that may be implicated in the rapid growth of some cancers.
The project, funded by the National Institutes of Health (NIH), is described in today's issue of the Proceedings of the National Academy of Sciences (PNAS).
"I was so amazed by what I was seeing," said lead author Masahiro Shuda, Ph.D., research assistant professor in Pitt's Department of Microbiology & Molecular Genetics. "We repeated and repeated our work to prove that the standard scientific dogma wasn't the complete story."
Dr. Shuda and his colleagues showed that a well-known cancer protein called mTOR, previously thought to be solely responsible for controlling a form of protein production important in cancer cells, called cap-dependent translation, can actually hand its work off to a different protein, CDK1, when cells are dividing. They observed the process while examining a viral oncoprotein that allows a common and usually harmless virus to transform healthy cells into cancer cells.
Merkel cell polyomavirus (MCV) was discovered in 2008 by co-authors Yuan Chang, M.D., and Patrick S. Moore, M.D., M.P.H., in the Cancer Virology Program at UPCI, partner with UPMC CancerCenter. It causes a rare but deadly skin cancer called Merkel cell carcinoma. They later found a viral protein called "small tumor protein," or sT. It may start a chain reaction that enables tumor growth resistant to cancer drugs that inhibit the protein mTOR.
In studies dating back to the 1960s, scientists had assumed that cap-dependent protein synthesis was turned off during cell division. The new study reveals that this is not necessarily so and that CDK1 can substitute for mTOR. Both mTOR and CDK1 work by inhibiting a gatekeeper protein, called 4E-BP1, that shuts off cap-dependent protein synthesis.
Less than 1 percent of cells are in the active division cycle called mitosis, even in very aggressive cancers, which makes studying cells in mitosis difficult. In addition, a drug traditionally used to arrest the cells during division inhibits protein production by CDK1. This is likely why previous research did not identify the important role that CDK1 appears to play.
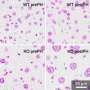
Dr. Shuda used a technology called flow cytometry to identify cells undergoing division. With special fluorescent tags, he was able to see mitotic cells produce fully inactivated 4E-BP1 by CDK1. He also directly measured proteins being made during mitosis.
Sure enough, even when mTOR was knocked out, CDK1 was still present and able to allow protein synthesis needed for cell division to progress.
"Now, we still can't say that this process involving CDK1 contributes to cancer - that's something we'll tackle with future research," said Dr. Moore, senior author and professor of molecular genetics and biochemistry at Pitt. "But it does point toward a fundamental control mechanism in cell biology and leads to the interesting possibility that creating or combining cancer drugs, so that they inhibit both mTOR- and CDK1-related protein synthesis, could be a very useful therapy to pursue."
More information: CDK1 substitutes for mTOR kinase to activate mitotic cap-dependent protein translation, PNAS, www.pnas.org/cgi/doi/10.1073/pnas.1505787112