Novel regulator inhibits toxic protein aggregates in Huntington's disease
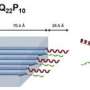
Huntington's disease is a neurodegenerative disorder characterized by huntingtin protein aggregates in a patient's brain, but how these aggregates form is not well understood. In a study published online today in Genome Research, researchers developed a novel computational strategy to identify interaction partners of the huntingtin protein and discovered a novel factor that suppresses misfolding and aggregation.
Huntington's disease is caused by an expansion of glutamine residues in the huntingtin protein, altering its function and ultimately resulting in toxic aggregation of huntingtin fragments in neurons. Proteins that interact with the glutamine-expanded huntingtin protein are thought to strongly influence the formation of the aggregates.
"The challenge that remains is if there are many proteins interacting with the huntingtin protein, we cannot easily determine which are relevant for disease and which are not," said Erich Wanker from Max Delbrück Center for Molecular Medicine and corresponding author of the study.
By combining large datasets of protein-protein interactions and filtering by brain-specific gene expression in patients with and without Huntington's disease, the scientists narrowed potential interactors to 13 candidates, including 7 that are known targets in Huntington's disease.
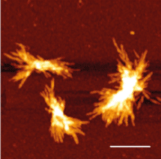
The researchers followed up on one candidate, CRMP1, because of its expression in brain and not elsewhere in the body. Using cell-based model systems and Drosophila, they found CRMP1 overexpression reduces hungtingtin aggregation and cellular toxicity, while reduced CRMP1 results in increased aggregation and toxicity. In cell-free assays, CRMP1 slows the spontaneous self-assembly of huntingtin fragments with glutamine expansions.
"CRMP1 was not regarded as a therapeutic target so far, now it is worth exploring as a potential target," said Wanker.
More information: Stroedicke M, Bounab Y, Strempel N, Klockmeier K, Yigit S, Friedrich RP, Chaurasia G, Li S, Hesse F, Riechers SP, Russ J, Nicoletti C, Boeddrich A, Wiglenda T, Haenig C, Schnoegl S, Fournier D, Graham RK, Hayden MR, Sigrist S, Bates GP, Priller J, Andrade-Navarro MA, Futschik ME, Wanker EE. 2015. Systematic interaction network filtering identifies CRMP1 as a novel suppressor of Huntingtin misfolding and neurotoxicity. Genome Res doi: 10.1101/gr.182444.114