Spinal stimulation system relieves pain without tingling
(HealthDay)—The Senza spinal cord stimulation system has been approved by the U.S. Food and Drug Administration to treat chronic back pain without the tingling sensation that characterizes more traditional pain-relieving methods.
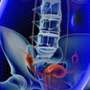
The implanted device uses high-frequency stimulation to avoid the tingling sensation known as "paresthesia," the agency said in a news release. Spinal pain could be characterized by conditions including failed back surgery syndrome, low back pain and leg pain.
Before treatment with Senza begins, potential users are treated with a trial system for a week or two, the FDA said. Once a physician determines that the trial device has worked, patients have minimally invasive surgery to implant Senza in the upper buttocks or abdomen. The device includes a patient-operated remote control.
Senza's safety and effectiveness were clinically evaluated in a study involving nearly 200 people. Three-quarters of those treated with Senza reported pain reduction of about 50 percent, the FDA said. The most common adverse reactions included pain at the implant site and dislocation of the device lead after implantation.
The system is manufactured by Nevro Corp., based in Menlo, Calif.
More information: Visit the FDA to learn more.
Copyright © 2015 HealthDay. All rights reserved.