Brain disease scenarios revised by step-by-step imaging of toxic aggregation
Diseases like Alzheimer's are caused when proteins aggregate and clump together. In a world first, EPFL scientists have successfully distinguished between the disease-causing aggregation forms of proteins. The finding can help change pharmaceutical treatment of neurodegenerative diseases.
Because of our increasing lifespan, diseases like Parkinson's, Huntington's and Alzheimer's are on the rise. They are caused when certain proteins misfold and aggregate together, forming clumps that damage neurons in the brain and spinal cord. This aggregation evolves progressively through different forms, which could hold the key to treating the diseases they cause. However, current imaging techniques have not been able to distinguish and study each aggregation form separately. Combining two advanced imaging techniques, EPFL scientists have successfully distinguished the different aggregation forms of a protein involved in spinocerebellar ataxia, which affects motor control and coordination. Published in Nature Communications, the work also reveals a surprising twist in protein aggregation, and can be extended to other diseases as well.
Aggregated problems
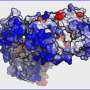
Before aggregating, single proteins misfold into anti-parallel or criss-crossed weave patterns. The misfolded proteins then begin to aggregate through intermediate form, ultimately growing into large stringy tangles called "fibrils". By this point, the aggregation has become lethal to the cells.
These protein aggregates are the focus of medical research in neurodegenerative diseases, including Parkinson's, Huntington's and Alzheimer's. The focus is on distinguishing between the individual aggregation form of the proteins, in order to identify which ones we can target more effectively to treat the diseases. However, current imaging techniques have not been able to distinguish between a protein's intermediate species and the final fibrils.
Untangling the knots
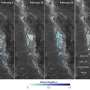
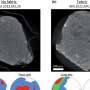
The lab of Giovanni Dietler at EPFL addressed the problem by combining two imaging techniques. The first, Atomic Force Microscopy, looks at the 3D structure and of each aggregation form, as well as its properties, e.g. stiffness. The second technique, Infrared Spectroscopy, detects the subtle changes that take place in the protein's structure that cause and drive its aggregation.
"These two techniques are very useful," says Dietler. "But individually, they cannot pinpoint the moment that the protein begins to misfold or find how the protein's structural properties relate to the structure of a particular aggregation form." Both these elements are critical in distinguishing different aggregation species. Together, the two techniques comprise an innovative tool known as "Infrared Nanospectroscopy", or nanoIR, which Dietler's lab spearheads in a range of applications.
In this case, the scientists focused on the protein ataxin-3. When it mutates, ataxin-3 begins to aggregate and form fibrils with devastating consequences on motor control and coordination. This neurodegenerative disease is known as spinocerebellar ataxia.
Using nanoIR, the EPFL researchers were able to monitor the evolution of individual ataxin-3 proteins as they aggregated. They looked at the stiffness of individual aggregated forms and then linked it to the number of weave patterns that they contained, providing a correlation between the two factors. It is the first time this has been done for individual aggregation forms.
A surprising twist
To their surprise, the nanoIR approach showed that ataxin-3 misfold after it aggregates, not before as would be expected by current views on aggregation. In fact, the aggregation of ataxin-3 seems to begin with the individual protein, and then moves onto the formation of intermediate aggregation forms with the original protein structure rather than a misfolded one.
There are significant medical and scientific implications of this finding. It finally confirms previous theories about protein misfolding that could not be tested due to the limitations of available techniques. At the same time it could change pharmacological and technological approaches to protein aggregation.
The study also demonstrates the enormous potential of nanoIR in this area of research. "It can allow us to get a deeper understanding of protein aggregation," says Giovanni Dietler.
More information: Ruggeri FS, Longo G, Faggiano S, Lipiec E, Pastore A, Dietler G. Infrared nanospectroscopy characterization of oligomeric and fibrillar aggregates during amyloid formation. Nature Communications 28 July 2015. DOI: 10.1038/ncomms8831
Journal information: Nature Communications
Provided by Ecole Polytechnique Federale de Lausanne