Controlling polymorphisms using lasers for pharmaceutical synthesis
Research reported in Applied Physics Express (APEX) by Kenji Ikeda and co-workers describes a new technique using lasers to induce selective crystallization of the metastable form of indomethacin. These results have potential applications for the synthesis of active pharmaceutical ingredients that can change their form for the development of therapeutic drugs.
Active pharmaceutical ingredients that can change their form – a trait known as polymorphism – are highly sought after in drug development. Different polymorphic forms of the same drug display different characteristics – for example, the metastable phase of the anti-inflammatory drug indomethacin is more soluble than the stable phase. Better solubility enhances how much of a dose circulates effectively in the body after administration, so retaining indomethacin in its metastable phase is therefore desirable. However, scientists have found this difficult to achieve because the drug naturally wants to revert to its dominant stable phase.
Kenji Ikeda and co-workers at Osaka University, together with scientists across Japan, have developed a new technique using lasers to induce selective crystallization of the metastable form (or α-form) of indomethacin. Their method creates α-form indomethacin which remains stable in air at room temperature for up to eight months: previous research achieved α-form stability for less than a day.
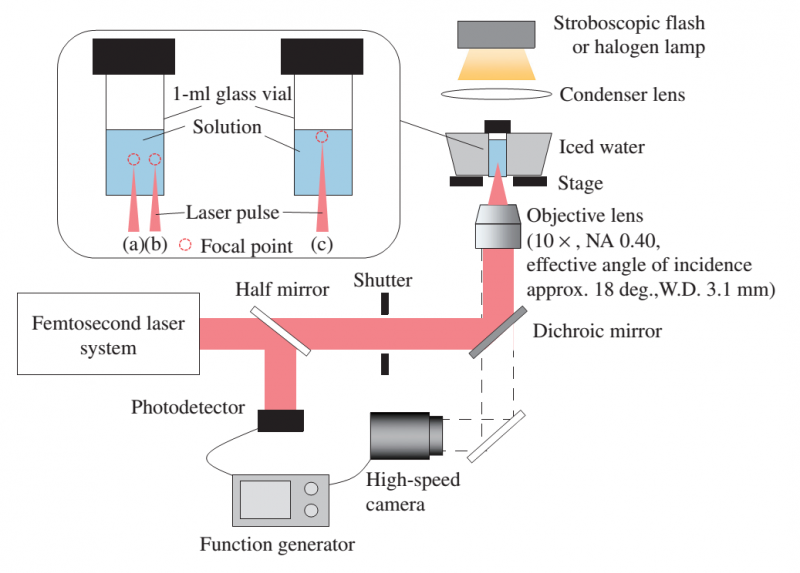
Metastable indomethacin crystallizes when 'supersaturated' in solution. Ikeda and his team generated 'captivation bubbles' in acetonitrile-indomethacin solution using laser irradiation, which enhanced the nucleation and crystallization of the metastable phase. The researchers found that, when the laser was focused near the side wall of the glass vials, the α-form of the drug crystallized rapidly and retained stability. The solubility of the new α-form was 1.5 fold higher than indomethacin's stable phase.
Ikeda and colleagues conclude laser irradiation at the solid-liquid interface is a highly efficient way of creating stable α-form indomethacin.
More information: "Selective crystallization of the metastable phase of indomethacin at the interface of liquid/air bubble induced by femtosecond laser irradiation." 2015 Appl. Phys. Express 8 045501 DOI: 10.7567/APEX.8.045501
Provided by Japan Society of Applied Physics