Three Alzheimer's genetic risk factors linked to immune cell dysfunction
People with a variant copy of the TREM2 gene have an increased risk of developing Alzheimer's disease, but researchers are only beginning to understand why.
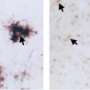
A Genentech study has uncovered details of how a type of immune cell helps the brain get rid of the tiny amyloid-beta aggregates that can clump together to form the plaques characteristic of Alzheimer's. The researchers, reporting July 20 in Neuron, found that TREM2 mutations can derail the immune cell's plaque-clearing activity, as can two other genes already known to increase a person's risk for Alzheimer's: APOE and APOJ (known as clusterin).
"I think we're only scratching the surface of what TREM2 does," said Morgan Sheng, a senior author of the paper and Vice-President of Neuroscience at Genentech.
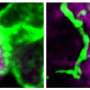
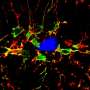
In healthy people, immune cells called microglia patrol the brain, surrounding and engulfing potential threats. Research published in May found that mice with a mutation in the TREM2 gene have microglia that can't efficiently surround amyloid deposits1. Scientists know that the TREM2 gene codes for a receptor protein located on the surface of microglia and when certain molecules bind to TREM2, it can stimulate microglia activity.
In their study, Sheng and colleagues ran an unbiased protein microarray screen of 1,559 extracellular proteins to see which ones might bind and interact with TREM2. A select group of lipoproteins were found to bind to TREM2, including LDL (low density lipoprotein) and the apolipoproteins APOE and APOJ (both risk factors in Alzheimer's disease).
"Lipoproteins float around in the blood, and their purpose is to carry cholesterol or lipids from one cell to another—as we all know, too much LDL is associated with high cholesterol and increased risk of cardiovascular disease," says Sheng. "Lipoproteins also exist in the brain, but less is understood about their role there."
Using cells purified from mice, the researchers explored how microglia reacted to amyloid-beta aggregates in different conditions. The team found that microglia could engulf amyloid-beta aggregates much more efficiently in the presence of LDL and APOJ, because the lipoproteins formed complexes with the amyloid-beta aggregates. The uptake of the lipoprotein-amyloid beta complexes by microglia depended on TREM2.
"It was a surprise that amyloid-beta was much more efficiently engulfed when bound in a lipoprotein complex, rather than when amyloid-beta aggregates were free and naked," says Sheng.
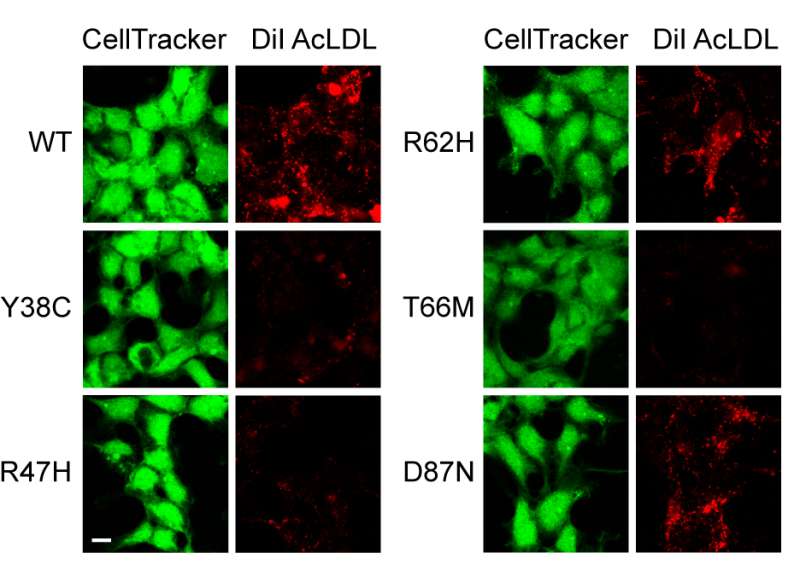
In another experiment, researchers tested blood samples of volunteers to see how variants of the TREM2 gene could change the way that human cells handled lipoproteins and amyloid-beta. While they couldn't get microglia from volunteers, they could test TREM2 found on the surface of macrophages, which are immune cells similar to microglia but can be obtained from blood.
They found that macrophages from people carrying a TREM2 variant associated with Alzheimer's disease had a diminished ability to engulf lipoprotein-amyloid beta complexes. And the researchers found that it only takes one variant copy of the TREM2 gene—not two—to impair this ability.
"Overall, these studies further point towards microglia as playing an important role in the pathogenesis of Alzheimer's disease," says Sheng.
Sheng hopes future studies will extend beyond the culture dish to confirm the activity of TREM2 on lipoproteins and amyloid-beta clearance in the brain.
More information: Neuron, Sheng et al.:"TREM2 binds to apolipoproteins including APOE and APOJ and thereby facilitates uptake of amyloid-beta by microglia" www.cell.com/neuron/fulltext/S0896-6273(16)30292-6 , DOI: 10.1016/j.neuron.2016.06.015
1 Peng Yuan et al, TREM2 Haplodeficiency in Mice and Humans Impairs the Microglia Barrier Function Leading to Decreased Amyloid Compaction and Severe Axonal Dystrophy, Neuron (2016). DOI: 10.1016/j.neuron.2016.05.003