Clinical trial tests cord-blood cells to treat macular degeneration
UIC is part of a national phase 2 clinical trial to evaluate the safety and tolerability of using cells derived from multipotent umbilical cord cells to treat age-related macular degeneration, the most common cause of vision loss in people over 55.
The disease causes light-sensitive cells in the center of the retina to degrade, leading to loss of central vision and the ability to read, drive and recognize faces.
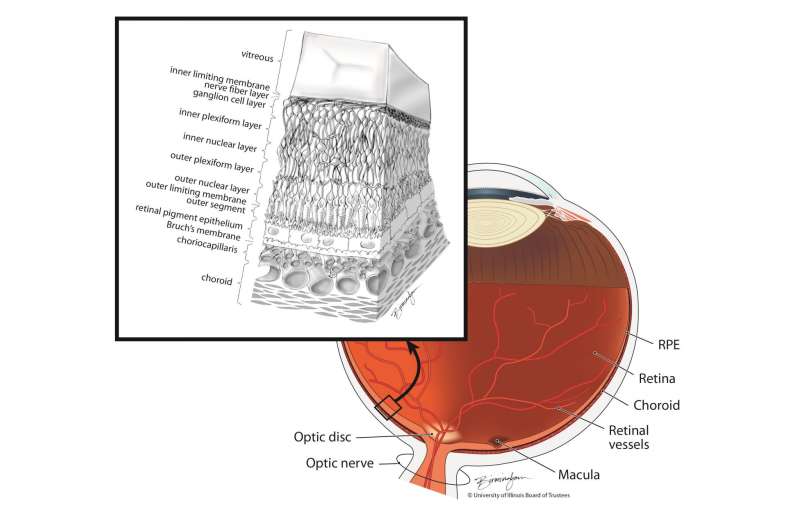
The cell therapy under investigation is for the "dry" form of age-related macular degeneration, which accounts for 90 percent of cases and currently has no treatment. Slower progressing than the wet form, it can ultimately lead to profound vision loss caused by a breakdown or thinning of the layer of retinal pigment epithelial cells in the macula. RPE cells form a layer just underneath the light-sensitive rod and cone cells of the retina and help nourish and support them.
In the experimental treatment, multipotent cells from umbilical cord blood are coaxed into differentiating into RPE-like cells in the lab. Doctors then inject the RPE-like cells under the retina, in the hope that they will prevent further loss of rod and cone cells—and perhaps even restore vision.
"If the treatment is successful, that would mean that we might be able to use it in people with the beginning stages of dry age-related macular degeneration, when vision loss is not so severe, in order to slow the loss of RPE and photoreceptor cells," said Dr. Yannek Leiderman, assistant professor of ophthalmology in the UIC College of Medicine and lead surgeon in the clinical trial at UIC.
Leiderman helped develop a specialized catheter to inject the RPE cells under the retina.
"Eye surgeons usually work within the vitreous cavity, or the center of the eye," Leiderman said. "To get behind the retina, we needed a totally new kind of catheter that could reach the area behind the very delicate retina without causing any collateral damage."
As part of the trial, in June, Leiderman injected the RPE-like cells into just one eye on each of two patients at UIC who have advanced dry age-related macular degeneration and significant vision loss. They will be followed up for several years.
A larger, phase 3 trial, in which participants will receive either cord cells or a placebo, will be needed to determine efficacy.
"It will still be quite a while before we can determine if the procedure has had an impact on vision," Leiderman said.
Dr. Paul Chan, professor of ophthalmology in the UIC College of Medicine, is a principal investigator on the trial.
More information: The clinical trial is funded by Janssen Cell Therapy.