Researchers develop computer simulation of body's heat response
For the first time, scientists at the M.M. Shemyakin and Yu. A. Ovchinnikov Institute of Bioorganic Chemistry of the Russian Academy of Sciences have successfully used computer-generated simulations to study the TRPV1-receptor temperature activation phenomenon for higher organisms. The results of the experiment were published in Scientific Reports.
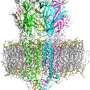
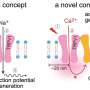
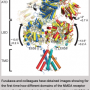
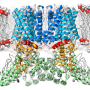
Belonging to the TRP-receptor protein group, TRPV1 participates in the body's temperature regulation system. Toward the end of the last century, an American scientist from the University of California in San Francisco, David Julius, discovered the thermoregulatory function of this ion channel, which, when the temperature rises, starts to pass sodium, magnesium and calcium ions through the cell membrane, while at the same time sending a signal to the cell to change the surrounding conditions. Later, Professor Julius decoded the TRPV1-receptor spatial structure using the cryo-electron microscopy method, which can detect a small-sized object (10-15 nm) that is frozen under high-pressure. The resulting structure was defective in that the statically frozen receptor partially made it impossible to decode any dynamic changes. Therefore, to date, the receptor activation process remains somewhat unclear. Anton Chugunov, one of the authors of the articlev, together with colleagues from the Institute of Bioorganic Chemistry, managed to fill this gap.
Until recently, it was impossible to achieve such results in in silico experiments due to imperfections in the computer simulation models. "We began this research study when our colleagues from the Laboratory of Neuroreceptors and Neuroregulators forwarded a request to study the dynamics and properties of the TRPV1-Receptor for the purposes of creating new anti-inflammatory and analgesic drugs," explains Chugunov. "During the course of the computations, we discovered that we could observe something more – the effect of the receptor activation temperature. This is a very delicate process, and molecular dynamics simulation, which allows us to study the 'behavior' of proteins, is still such an inaccurate 'molecular microscope' that we were amazed by the possibilities that opened before us."
"We took two states of the receptor's computer model: open and closed," says Chugunov. "To see how it worked, we carried out molecular dynamics calculations, which were quite impressive, lasting a year, and also used the pore 'computer-mapping' method. We 'unwrapped' the pore—that is, projected the properties of its internal channel-forming surface onto a plane, and clearly visualized them at different temperatures. Using a specially developed program, we monitored the changes in pore radius, which took place gradually over time, step by step, as happens in a living cell. The strong discontinuities that we registered on the graph indicated that the pore had changed its state from closed to open, but not vice versa. We knew that the receptor is activated at temperatures above 43 ° C, and anyone can feel this by placing his/her hand in hot water. We were therefore able to figure out the molecular dynamics for each state at four temperatures: two higher, and two lower than 43 ° C. The results were surprising."
"We logically assumed that the receptor for which the molecular dynamics calculation had been launched under the closed channel pore must remain closed at the two lower temperatures and open at the two higher ones, and vice versa. However, the computational experiment showed that in the closed position, the channel pore opened at high temperatures, but during the calculation of the pore's open state, lower temperatures failed to close the pore for the duration of the computational time (up to 1 microsecond). The result that it is much easier to open the ion channel than close it was a kind of unbalance," says Anton.
The data published show that modern computing capabilities help in investigating the ion channels and their operating characteristics in time intervals that are still impossible to detect experimentally. The researchers were also able to observe the transitions within the receptor molecule as if through a 'molecular microscope'.
This work is ultimately designed to develop a future virtual TRPV1-receptor that is suitable for predicting the structure of selective ligands (special molecules working with proteins). The researchers have reason to expect that such ligands could find application in medicine as an analgesic medication. In this case, they would eliminate most of the side effects that are peculiar to modern medicines of the nonsteroidal anti-inflammatory drug groups.
More information: Anton O. Chugunov et al. Temperature-sensitive gating of TRPV1 channel as probed by atomistic simulations of its trans- and juxtamembrane domains, Scientific Reports (2016). DOI: 10.1038/srep33112
David Julius et al. , Nature (1997). DOI: 10.1038/39807