Study shows promising clinical activity
Immune cellular therapy is a promising new area of cancer treatment. Anti-cancer therapeutics, such as chimeric antigen receptor (CAR) modified T cells, can be engineered to target tumor-associated antigens to attack and kill cancer cells. This allows for an improved precision medicine approach to treating cancer. Moffitt Cancer Center physician-scientist Fredrick L. Locke, M.D., will present interim results from cohort 2 of the phase 2 portion of the ZUMA-1 study, which uses CAR-T therapy for patients with refractory primary mediastinal B-cell lymphoma and transformed follicular lymphoma, during the American Society of Hematology Annual Meeting in San Diego.
The ZUMA-1 study utilizes KTE-C19 which is an autologous chimeric antigen receptor (CAR) T cell therapy. During KTE-C19 CAR-T therapy, T cells are isolated from a patient's blood and genetically engineered in Kite's laboratory to target the CD19 protein that is found on lymphoma cells. The genetically engineered T cells are then infused within the patient that they were originally harvested from. The KTE-C19 T cells are able to recognize cancerous lymphoma cells that express CD19 and target them for destruction.
The phase 1 portion of the ZUMA-1study revealed that KTE-C19 was tolerable and produced ongoing remissions in B-cell non-Hodgkin lymphoma patients (Locke et al, Abstr. 1048O, ESMO Congress 2016). The phase 2 portion of the study consists of two cohorts: cohort 1 for patients with chemo-refractory Diffuse Large B cell Lymphoma; and cohort 2 for patients with chemo-refractory primary mediastinal B-cell lymphoma or transformed follicular lymphoma.
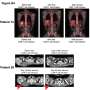
As of June 16, 6 patients with refractory primary mediastinal B-cell lymphoma or transformed follicular lymphoma were treated in cohort 2 with KTE-C19. The patients were highly refractory to prior treatments and included 3 patients who were refractory to second-line or greater therapy and 3 patients who relapsed after autologous stem cell transplants.
KTE-C19 therapy resulted in promising clinical activity in these patients. With a median follow-up period of 3.2 months, all 6 patients achieved a complete remission. KTE-C19 treatment also resulted in manageable toxicities that were generally reversible. Grade 3 treatment-emergent adverse events occurred in 17 percent of patients and grade 4 treatment-emergent adverse events occurred in 67 percent of patients. All 6 patients experienced cytokine release syndrome; however, all cases were grade 1 or 2. Neurotoxicity occurred in 67 percent of patients and 33 percent of the cases were grade 3.
Locke, who is an assistant member of Moffitt's Blood and Marrow Transplantation Department and co-lead principal investigator of the global ZUMA-1 multicenter trial, will present updated phase 2 cohort 2 ZUMA-1 interim results Monday, Dec. 5 at 3 p.m. in room 24 of the San Diego Convention Center.