Research reveals how physical exercise protects the heart
Regular exercise is considered an important form of treatment for heart failure, a condition in which the heart is unable to pump enough blood to meet the body's needs. The benefits of exercise include prevention of cachexia, control of arterial blood pressure, and improved cardiac function, postponing a degenerative process that causes progressive heart cell death. About 70 percent of heart failure patients die from the condition within five years.
A study by the University of São Paulo (USP) in Brazil, published recently in the journal Autophagy, elucidates part of the mechanism whereby aerobic exercise protects the sick heart. "Basically, we discovered that aerobic training facilitates the removal of dysfunctional mitochondria from heart cells," said Julio Cesar Batista Ferreira, a professor at the Biomedical Science Institute (ICB-USP) and principal investigator for the project.
Mitochondria are the organelles in charge of providing energy to cells. "The removal of dysfunctional mitochondria increases the supply of ATP [adenosine triphosphate, the molecule that stores energy for the cell] and reduces the production of toxic molecules, such as oxygen free radicals and reactive aldehydes, an excess of which damage the cell structure," he said.
According to Ferreira, the long-term aim of the research is to identify intracellular targets that can be modulated by drugs to produce at least some of the cardiac benefits obtained by means of physical exercise. "We don't aim to create an exercise pill, which would be impossible, because exercise acts at many levels and throughout the organism. But it might be feasible for a drug to mimic or maximize the positive effect of physical activity on the heart," Ferreira said.
In a previous study, published in PLOS ONE, the group showed through experiments with rats that aerobic training reactivates the proteasome, an intracellular complex responsible for cleansing cells of damaged proteins. The results also showed that proteasome activity in the heart of a patient with heart failure decreases by more than 50 percent, and as a result, highly reactive proteins build up in the cytoplasm, where they interact with other structures and cause heart cell death.
In the article, which was featured on the journal's cover, the group showed that exercise also regulates the activity of another cellular cleansing mechanism, known as autophagy, the discovery of which led Japanese scientist Yoshinori Ohsumi to win the 2016 Nobel Prize in Medicine. "Instead of degrading isolated proteins, this system creates a vesicle [autophagosome] around dysfunctional organelles and transports all this material to the lysosome, a sort of incinerator," Ferreira explained. "The lysosome contains enzymes that destroy cell waste. However, we observed that autophagic flux is interrupted in the heart of a rat with heart failure and that there's a buildup of dysfunctional mitochondria."
The mitochondria may even divide to isolate the damaged part and facilitate its removal, he added. The researchers were able to observe this by analyzing the activity of proteins related to the process of mitochondrial division. However, the system that should transport the rejected mitochondria to the lysosome is unable to complete the task.
Experiments
The experimental rat model, which was the same as in the previous project, consisted of tying off (ligating) one coronary artery to induce a heart attack (myocardial infarction). Lack of cardiac blood irrigation caused the immediate death of some 30 percent of heart cells. After a month, the animals displayed signs of heart failure.
When the researchers analyzed tissue from the animals' hearts under an electron microscope at 3,000 times magnification, they found that the cells contained large clusters of small fragmented mitochondria. This was not observed in the group of healthy rats. These mitochondria were placed in an apparatus that measured oxygen consumption and hence assessed mitochondrial metabolism. The test confirmed that mitochondrial respiration was not functioning properly.
"The images showed that membranes were trying to form around these small mitochondria, but the autophagosome failed to surround them completely," Ferreira said. "We concluded that they were accumulating because the removal system wasn't working. The rats were placed on the treadmill, and the dysfunctional mitochondria disappeared. The exercise restored the process of dysfunctional cardiac mitochondria removal. The benefits of exercise were abolished when we blocked autophagy pharmaceutically or genetically."
Training of the animals began four weeks after induction of the heart attack, by which time they were displaying signs of heart failure. They were placed on a treadmill running at moderate intensity (60 percent of maximum capacity) for 60 minutes once a day, five days a week, for eight weeks. At the end of this period, the results were compared with those of rats with heart failure that had remained sedentary and a control group of sedentary rats without induced heart failure.
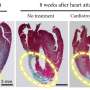
"In the sick rats that remained sedentary, cardiac function fell by 30 percent in the eight-week period, whereas in the trained group, it increased by 40 percent compared with the pre-training condition," Ferreira said. "Therefore, the difference between these two groups in terms of cardiac function was 70 percent."
The hearts of the sedentary rats with heart failure were 18 percent larger on average than those of the control group, whereas those of the trained rats were only 5 percent larger. "Physical exercise does increase the size of the heart, but this increase is proportional to the improvement in cardiac function. The dilation caused by heart failure relates to loss of cardiac function," Ferreira said.
ATP levels in the sedentary rats with heart failure were 50 percent lower than in the control group, but in the trained animals, they were equivalent to those of healthy hearts. "So our results show that exercise not only prevents but also reverses the damage caused by heart failure," Ferreira said. "Our hypothesis is that physical training modulates the expression and/or activity of one or more key proteins involved in mitophagy, or mitochondrial autophagy, thereby restoring its activity. We're now trying to confirm this hypothesis."
Once identified, he added, these genes and the proteins encoded by them could be tested as therapeutic targets.
A simpler model
As Ferreira explained, discovering the impact of every gene and protein on the cardiac adaptations deriving from physical activity in organisms as complex as mammals would be a huge task and probably impossible. For this reason, the roundworm Caenorhabditis elegans serves as a model for the group's ongoing research.
"Although this roundworm is a far less complex organism, as much as 90 percent of its genome is the same as ours for some protein families," he said. "Moreover, we can use existing tools such as functional genomics for large-scale assessment of each gene's contribution to adaptive responses to adverse conditions. The idea is to characterize the functional impact of the genes involved in mitochondrial division and mitophagy on the adaptations deriving from physical exercise." The challenge now is to validate a methodology for use in exercise training for worms.