Vulnerability identified for subtypes of glioblastoma
Glioblastoma, the most common and aggressive form of brain cancer, typically fails to respond to treatment or rapidly becomes drug resistant. In a paper published online in the journal Cancer Cell on November 30, University of California San Diego School of Medicine researchers identified a strategy that pinpoints a genetically distinct subpopulation of patients with glioblastoma that is particularly sensitive to drugs like cilengitide that target a cell adhesion receptor known as integrin αvβ3.
Cilengitide was developed based on early studies by David Cheresh, PhD, Distinguished Professor of Pathology at UC San Diego School of Medicine, and colleagues who demonstrated that αvβ3 expression was linked to the progression of glioblastoma. The drug was tested in clinical trials but production was halted in 2014 when it failed to show significant improvement in overall survival among participants during phase III trials.
"In early trials, cilengitide showed promise as some patients in the trial seemed to respond to the drug and appear to show extended survival," said Cheresh, associate director of innovation and industry alliances at UC San Diego Moores Cancer Center. "We questioned why these patients responded while most did not. We now know that the people who responded to cilengitide have a unique genetic signature that makes them highly susceptible to this drug. More importantly, we have a molecular understanding as to why these patients respond to this drug."
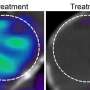
Cheresh and an international team of scientists found cilengitide is effective in patient-derived tumor cells of a defined subpopulation with proneural and classical glioblastoma subtypes. The Achilles' heel for these tumors is their addiction to the expression of the high affinity glucose transporter Glut3—a known driver of cancer stem cells.
"Dependency on Glut3 is critical—a mere expression of this protein is insufficient. What we saw is that many tumors are not addicted to Glut3 and that's why response to cilengitide was limited," said Cheresh. "We found that about 15 percent of patient-derived glioblastoma stem cell models were addicted to Glut3 and therefore attacking these tumors with an αvβ3 inhibitor like cilengitide was effective."
Cancer stem cells thrive on Glut3, which helps tumor cells take up glucose even in very low glucose environments, such as the brain. Integrin αvβ3 increases glucose uptake in the brain by enhancing Glut3 expression, which is critical for tumor growth among this subpopulation of patients, which is why cilengitide appears to be effective for these tumors.
Armed with this new information, the team reported that, utilizing a gene profile alone, they were able to successfully predict which glioblastoma tumors were sensitive to αvβ3 blockade. Currently, there are a number of αvβ3-targeted strategies in development that could be harnessed to target this vulnerability.
This year, more than 12,000 Americans will be diagnosed with glioblastomas, according to the American Brain Tumor Association. Among them: U.S. Senator John McCain, who announced his diagnosis in July. They are highly malignant. The two-year survival rate is 30 percent.
Standard treatment is aggressive: surgery, followed by chemotherapy and radiation. Yet most tumors recur within six months, fueled by a small population of glioblastoma stem cells that resist and survive treatment, continuing to divide and produce new tumor cells to replace those killed by cancer drugs.
"Using a precision medicine approach, we should be able to look at the genetic signature of a patient's glioblastoma to identify in advance if the patient will respond to this therapeutic approach. An estimated 15 percent of patients may see a benefit," said Cheresh. "With an advanced understanding of glioblastoma and cancer stem cells, we now have a therapeutic target and the ability to selectively treat tumors that are susceptible to this intervention."
While not all glioblastoma patients will benefit, these findings represent a major step in identifying a distinct subpopulation who might respond to this type of drug. The next step would be a clinical trial in which patients were selected based on their genetic profile, said Cheresh. The authors believe this drug might be useful in other types of cancer patients with a similar vulnerability.
More information: Érika Cosset et al, Glut3 Addiction Is a Druggable Vulnerability for a Molecularly Defined Subpopulation of Glioblastoma, Cancer Cell (2017). DOI: 10.1016/j.ccell.2017.10.016