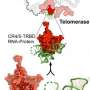
Scientists create a 3-D model of molecules in yeast linked to enzyme that lengthens chromosome tips
Through the haze of a sonogram screen, an expectant mother catches a glimpse of the growing baby within her. The outline of a nose, chin and head, instantly recognizable as a tiny human, brings to life what parents, until then, could only imagine. Biologists, too, aim to bring their scientific discoveries to life by creating three-dimensional models—at the atomic level—of the inner workings of cells.
"We need atomic-resolution 3-D images of molecular structures for many reasons. For example, these images can show us precisely how interacting molecules bind to each other in order to carry out critical cellular functions. This helps us develop therapeutic drugs that control the interactions, and therefore also the biochemical processes that they perform in cells," says David Zappulla, Ph.D., a researcher in the department of molecular biology and genetics at the Johns Hopkins University School of Medicine.
Zappulla's research focuses on an enzyme found in cells, called telomerase, which lengthens repetitive bits of DNA at the end of chromosomes. These end-caps, called telomeres, erode each time a cell divides, and without these protective tips, this erosion would chip away at the chromosomes—including crucial genetic information—and kill the cell.
Telomerase is present in fetal cells to keep DNA from getting too clipped as cells multiply rapidly during early development, but then the enzyme is turned off, and telomeres erode over time, as part of the natural aging process of cells. It's well-known that older people tend to have shorter telomeres than younger people.
Cancer cells, on the other hand, hijack telomerase and re-express it to maintain telomere length, making them impervious to aging-related death. To kill cancer cells, scientists have long sought drugs that target telomerase's ability to keep cells alive.
But to develop such drugs, scientists need a better understanding of how telomerase gets to and extends the chromosomes' ends.
"There appear to be multiple regulatory steps that precisely control telomerase and recruit it to the shortest chromosome ends where and when it's needed," says Zappulla, who has worked to reveal these processes. He published research in 2015 showing how two proteins, Ku and Sir4, interact to lure telomerase near the tips of yeast chromosomes.
In experiments looking at telomerase in baker's yeast, his lab showed that the Ku protein helps telomerase sense when a telomere is short. They showed that Ku binds to another protein, Sir4, and this connection is important for telomere lengthening. He believes that Sir4 acts as a landing pad to attract telomerase preferentially to short chromosome tips that need an extension.
To visualize these concepts in 3-D, Zappulla teamed up with Ming Lei, Ph.D., an expert in creating crystal structures, at the Shanghai Jiao Tong University. The two met during their postdoctoral training at the University of Colorado Boulder.
For the current research, published Jan. 11 in Cell, Lei's team crystallized the baker's yeast versions of key telomerase-recruiting proteins, as well as a piece of the telomerase enzyme's RNA. Then they shot X-rays through the crystals and inferred the 3-D shape of each molecule based on how the X-rays' paths are redirected. Then several co-teams collaborated to validate the structures by introducing mutations in the genes encoding the proteins and testing the altered molecules' functions in live yeast cells. These experiments led to new insights into how telomerase-recruiting proteins work and interrelate in time and space.
"It's amazing how much precise detail you can get from crystallography studies," says Zappulla.
When Zappulla first saw the results, he says that they immediately answered one of his questions about how telomerase interacted with Ku and Sir4 to attach to the chromosome end. "The crystal structures show how Ku binds to both the RNA in telomerase and the Sir4 protein on the chromosomes, as we had proposed in our 2015 study."
Zappulla says that yeast telomerase and the way it works will certainly be different than the human version; however, insights from yeast should help scientists understand fundamental molecular and cellular features that are similar or even have been conserved over evolution.
More information: Hongwen Chen et al. Structural Insights into Yeast Telomerase Recruitment to Telomeres, Cell (2017). DOI: 10.1016/j.cell.2017.12.008