The subcellular dynamics of RNA stabilizing molecule in response to inflammation
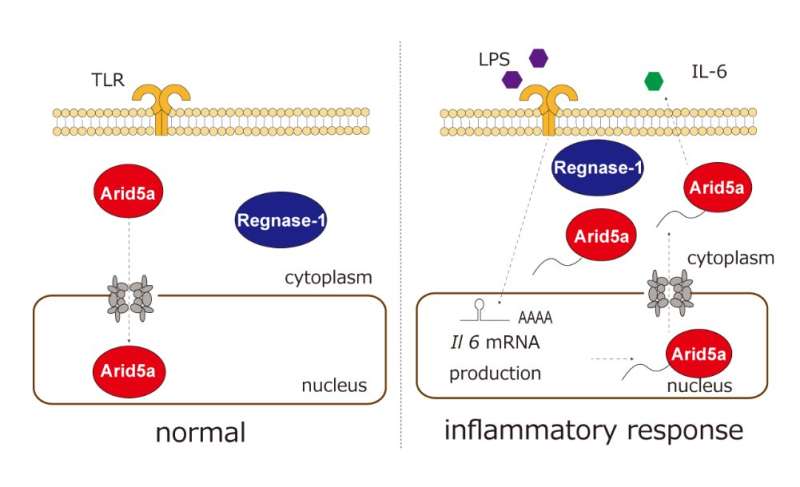
A research group at Osaka University revealed the regulatory mechanism of subcellular localization of Arid5a in response to inflammation. It has been known that an inflammatory accelerator is localized in the nucleus, and an inflammatory brake is localized in the cytoplasm.
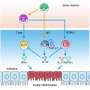
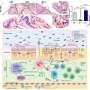
Tadamitsu Kishimoto (Professor, Immunology Frontier Research Center (IFReC), Osaka University) and histheir research group revealed the regulatory mechanism of subcellular localization of Arid5a in response to inflammation. It has had been known that an inflammatory accelerator, Arid5a, is localized in the nucleus, and an inflammatory brake, regnase) , is localized in the cytoplasm. In this study, they showed that 1) Arid5a translocates to the cytoplasm from the nucleus in response to inflammation, 2) bimax, which inhibits cNLS-dependent nuclear import via high-affinity interactions with NLS-binding sites of importin-α, inhibits the nuclear import of Arid5a, and 3) a CRM1 inhibitor, Leptomycin B, inhibits the nuclear export of Arid5a after LPS stimulation.
Macrophages produce inflammatory cytokines to activatee other immune cells and to exclude pathogens. However, over- or chronic inflammation causes diseases including a septic shock or autoimmunity. Therefore, the group has been studiedstudied the control mechanism of inflammation, especially focusing on the posttranscriptional regulation of the Il6 mRNA by Arid5a and Regnase-1. Their previous study showed that Arid5a binds to Il6 mRNA 3′UTR to inhibit Regnase-1-mediated
RNA decay. Additionally, Arid5a deficient mice showed down-regulated inflammatory cytokine production, resistance to septic shock, and bleomycin-induced lung injury. Although Arid5a is known to play an important role in immune regulation, whether and how Arid5a subcellular localization impacts immune regulation has remained unclear.
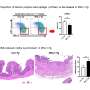
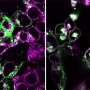
The group showed that Arid5a translocates from the nucleus to the cytoplasm after LPS stimulation. Since the inhibition of Arid5a nuclear export causes the significant suppression of IL-6 production, further understanding of Arid5a dynamics may lead to novel therapeutic strategies of for septic shock or autoimmune diseases.
More information: Mitsuru Higa et al. Regulation of inflammatory responses by dynamic subcellular localization of RNA-binding protein Arid5a, Proceedings of the National Academy of Sciences (2018). DOI: 10.1073/pnas.1719921115