Gout drug reduces adverse events in patients with hyperuricaemia
Uric acid reduction with the gout treatment febuxostat reduces adverse events in elderly patients with hyperuricaemia, according to late breaking research presented today in a Hot Line Session at ESC Congress 2018.
Hyperuricaemia, an abnormally high serum uric acid level, causes gout and is associated with coronary artery disease, hypertension, stroke, renal failure, and death. The FREED study examined whether lowering uric acid with febuxostat prevents cerebral, cardiovascular and renal events and death in elderly patients with the condition.
The study enrolled 1,070 patients aged 65 years and older with hyperuricaemia (serum uric acid 7–9 mg/dL) and at risk for cerebral, cardiovascular, or renal events as defined by the presence of hypertension, type 2 diabetes, renal disorder, or a history of cerebral or cardiovascular disease.
Patients were randomly assigned in a 1:1 ratio to receive oral febuxostat for 36 months or not. In the febuxostat group, the dose was increased stepwise from 10 to 40 mg per day if tolerated. In the non-febuxostat group, allopurinol 100 mg was considered if serum uric acid was elevated. In both groups, the dose of febuxostat or allopurinol was adjusted to avoid a serum uric acid level less than 2 mg/dL. All patients were advised to consume a healthy diet, quit smoking, and exercise to help manage their hyperuricaemia.
Patients were followed-up for 36 months for the primary endpoint which was a composite of cerebral, cardiovascular, and renal events, and death from any cause. This consisted of death due to cerebral or cardiorenal vascular disease, new or recurring cerebrovascular disease, new or recurring coronary artery disease, cardiac failure requiring hospitalisation, arteriosclerotic disease requiring treatment, renal disease (development of microalbuminuria, progression to overt proteinuria, or worsening of overt proteinuria to ≥300 mg/g albumin/creatinine, doubling of serum creatinine level, progression to end stage renal disease defined as estimated glomerular filtration rate <15 mL/min/1.73 m2), and new atrial fibrillation.
A total of 537 patients received febuxostat and the average dose at the end of the study was 29 mg. Of the 533 patients in the non-febuxostat group, 27 percent received allopurinol 100 mg. Average serum uric acid levels reached 4.4 mg/dL in the febuxostat group and 6.7 mg/dL in the group not receiving febuxostat.
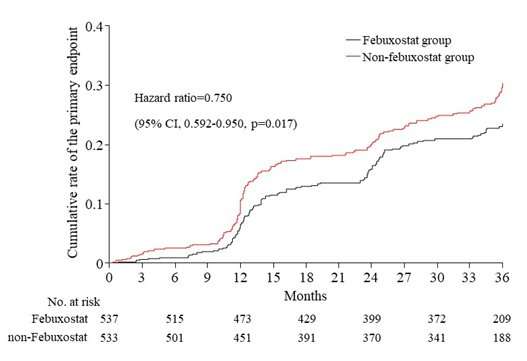
The primary endpoint occurred in 125 (23 percent) patients in the febuxostat group and 153 (29 percent) patients in the non-febuxostat group. Febuxostat significantly reduced the rate of the primary endpoint, with a hazard ratio of 0.75 (95 percent confidence interval 0.59–0.95, p=0.017) (see figure).
When the individual components of the primary endpoint were analysed separately, the most frequent event was renal impairment, which occurred in 16.2 percent of the febuxostat group and 20.5 percent of the non-febuxostat group. Among those with renal impairment, the development of microalbuminuria or mild proteinuria was common in both treatment groups.
Adverse events were observed in 132 (24.6 percent) patients in the febuxostat group and 135 (25.3 percent) patients in the non-febuxostat group, with no significant difference between the two groups (p=0.83). Two patients in the febuxostat group died. Also in the febuxostat group, mental disorder, headache, hypertension, abdominal pain, diarrhoea, maculopapular rash, acute kidney injury, and oedema of the extremities occurred in two patients each.
Professor Sunao Kojima, principal investigator, Kawasaki Medical School, Okayama, Japan, said: "We found that patients receiving febuxostat were 25 percent less likely to die or experience strokes, heart disease, or kidney disease over a three-year period than patients who did not receive febuxostat. The findings suggest that lowering uric acid with febuxostat provides clinical benefit."