Scientists find a new way to target norovirus family
MRC scientists have taken a major step forward in understanding how a family of viruses, including norovirus, initiate infections.
The new study, led by researchers at the MRC Centre for Virus Research at the University of Glasgow, reveals the inner workings of the calicivirus family, which includes norovirus and sapoviruses. These are highly infectious viruses that can cause outbreaks of diarrhoea and vomiting.
It is hoped this research may provide a new target for the development of antiviral drugs to prevent diseases like norovirus.
Norovirus is highly contagious and can be very difficult to contain. Commonly, outbreaks occur in hospitals, care homes, schools, hotels and on cruise ships. Caliciviruses are also important animal pathogens, causing 'cat flu' that can be associated with very high mortality rates in domestic cats.
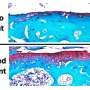
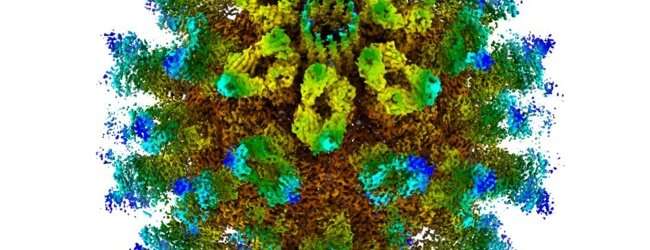
Using the Nobel prize-winning technique of Cryo-Electron Microscopy (Cryo-EM), MRC scientists studied the structure of the virus that causes 'cat flu' – feline calicivirus – to better understand how this family of viruses initiates infections.
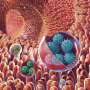
The scientists discovered that after binding to the cell surface, these viruses undergo structural changes leading to the formation of a portal – a funnel-shaped structure on the virus particle surface – which is thought to insert into the membrane of the cell, allowing the virus to inject its genome into the host cell and begin the infection process.
This insight into the early stages of calicivirus infection provides a new target for the development of antiviral drugs to tackle this family of viruses.
Dr. David Bhella, who led the research, said: "When viruses infect us, they bind to and then enter our cells. This is often by a process known as 'endocytosis', the process cells use to bring in nutrients from their environment."
Viruses trigger endocytosis, causing the cell to bring the virus particle into the cell in a bubble or vesicle called an 'endosome'. The virus then needs to break out of the endosome to release their genes into the cell and start the infection.
Dr. Bhella said: "Furthermore, we have calculated an atomic model of the portal protein—known as VP2. While VP2 was known to be critical for the production of infectious virus, its function has been hitherto undetermined. Our finding that VP2 assembles a portal that is likely responsible for endosome escape represents a major step forward in our understanding of both the Caliciviridae and icosahedral RNA containing viruses in general."
The full findings are published in the journal Nature.
More information: Michaela J. Conley et al. Calicivirus VP2 forms a portal-like assembly following receptor engagement, Nature (2019). DOI: 10.1038/s41586-018-0852-1