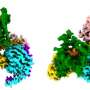
Key drug target shown assembling in real-time
Over one-third of all FDA-approved drugs act on a specific family of proteins: G-protein coupled receptors (GPCRs). Drugs to treat high blood pressure, asthma, cancer, diabetes and myriad other conditions target GPCRs throughout the body—but a recent study shows what happens next. In results published in Cell, researchers outline the timeline of events, including precisely when and how different parts of a GPCR interacts with its G protein signaling partners. The findings provide new insights into the fundamental mechanisms of drug-induced signaling in cells, including ways to identify the most critical portions of GPCRs for targeting development of novel therapeutics.
"We're able to see—from millisecond to minutes timescales—the detailed sequence of events where a GPCR encounters its downstream signaling partner and catalyzes a change in its structure, providing the basis for understanding its signaling," said corresponding author David Lodowski, PhD, assistant professor in the Department of Nutrition at Case Western Reserve University School of Medicine. "The most exciting part is that we can follow the signaling in a time-resolved manner. We first rapidly mix the activated GPCR and its G-protein signaling partner, and then capture time-resolved details along the natural signaling pathway."
The researchers observed the formation of the GPCR signaling complex using a powerful technique called "radiolytic footprinting" that couples chemical labeling of proteins with mass analysis. In this technique, high intensity X-rays are used to generate highly reactive chemical labels from the water surrounding proteins, enabling a "snapshot" of the protein's regions of interest. This x-ray footprinting technique was pioneered by Mark Chance, PhD, vice dean for research at Case Western Reserve University School of Medicine and a coauthor on the manuscript.
"Our footprinting approach efficiently labels the outside of proteins," Lodowski explained. "If a protein in isolation is labeled on one side, and then in complex is no longer labeled there, we know that's most likely the interaction surface." This approach helped the researchers understand how a GPCR uses its different parts of its surface to engage with the G protein.
Previous studies have shown what GPCRs look like at rest (before activation) and long after they've formed complexes with other proteins (and signaling is over). The in-between steps have been more elusive. Said Lodowski, "We are now moving into the fourth dimension—the temporal dimension—of how these complexes form."
Activated GPCRs form a complex with particular G proteins inside cells that control cell functions. The process transfers information (e.g. the signal) from the GPCR to the signaling partner. The study reveals specifics in this process, called the "G protein cycle." In milliseconds to seconds, GPCRs identify a signal (such as a hormone or drug), reconfigure themselves, recruit specific G proteins inside cells, and activate cellular signaling cascades.
The new analysis technique can identify when a certain portion of a GPCR—individual amino acids, for example—locks in with target amino acids inside a G protein. It thus reveals precise amino acids most central to GPCR function. If applied to GPCRs known to cause disease, such a detailed analysis could potentially uncover new sites for precision drug targeting. "We can use the same techniques to identify precise regions on GPCRs to target therapeutically," Lodowski explained. "If we know site A gets touched before site B in the cycle, then we can design better, more effective drugs."
GPCRs are not easy subjects to study. They are embedded in cell membranes, a natural location that facilitates their ability to transmit information from outside to inside the cell. However, this location complicates their isolation, purification and analysis. Due to these difficulties, structures of GPCRs and their complexes have been exceedingly difficult to solve, with the first structure of a GPCR only determined in 2000. Extensive GPCR structure determination efforts over the past 20 years have enabled the structure determination of a number of GPCRs, including the first GPCR-G protein complex structure in 2012, which earned Brian Kobilka, MD, of Stanford University the Nobel Prize in Chemistry. Kobilka was also a corresponding author on the new Cell publication.
"This work would not have been possible without our world-class team of investigators including collaborators at Stanford, Sungkyunkwan University in Korea, the University of Copenhagen in Denmark, and the scientists and engineers at the Case Center for Synchrotron Biosciences at Brookhaven National Laboratory," Chance said. The Case Center for Synchrotron Biosciences, located at the National Synchrotron Light Source II (NSLS-II) at Brookhaven laboratory, operates the custom footprinting beamline (BM-17) the researchers used in the new study.
Going forward, the researchers plan to use the beamline at NSLS-II to further analyze activation of GPCRs and their complexes. They'll combine their findings with existing GPCR structural data to better understand how GPCRs work. The results could lead to better beta-blockers, chemotherapy drugs, even drugs to treat vision or cognitive deficits.
"GPCRs are critical targets for a variety of new drugs," Lodowski said. "We've had some ideas of how GPCRs and their signaling complexes come together, but not the sequence of events with molecular detail. It's exciting that this novel time resolved approach allows us to extract more meaning from these structures."
More information: Yang Du et al, Assembly of a GPCR-G Protein Complex, Cell (2019). DOI: 10.1016/j.cell.2019.04.022