New strategy of reprogramming regulatory T cells may improve cancer therapies
While therapies that harness the power of the immune system against cancer have made remarkable progress against certain types of tumors, they still remain ineffective in most cancer patients. A new study from the Center for Immunology and Inflammatory Diseases (CIID) at Massachusetts General Hospital (MGH) describes a method of reprogramming the regulatory T cells that usually suppress immune responses into inflammatory cells that not only permit but also intensify an antitumor immune response. Their paper is receiving advance online release in Nature.
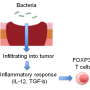
"Many patients' tumors do not respond to immune therapies—such as immune checkpoint blockade—because of a lack of pre-existing inflammation that is required for those therapies to work," says Thorsten Mempel, MD, Ph.D., of the MGH CIID, senior author of the Nature paper. "Our study shows that reprogrammed Treg cells provide exactly the type of inflammation that is lacking. Indeed, we found in mice that reprogramming tumor-infiltrating Treg cells to secrete inflammatory cytokines makes previously unresponsive tumors highly sensitive to PD-1 blockade."
The MGH study focused on the CBM complex—a large protein cluster within immune cells that helps regulate their activation, proliferation and function. Recent research has revealed a critical role for the CBM complex in lymphocyte function, and since deleting one of three key proteins, called CARMA1, is already known to reduce the function of effector T cells, the team examined the effects of CARMA1 deletion on Treg cells.
Their experiments revealed that targeting the CBM complex—either by deleting one or both copies of the CARMA1 gene in Treg cells or by treating tumor-bearing mice with a drug that inhibits MALT1, another component of the complex—caused Treg cells to secrete the immunostimulatory cytokine interferon gamma in tumor tissue alone. The ability to selectively modulate the function of Treg in tumors can avoid the risk of autoimmune disease that would result from systemic Treg depletion.
CBM targeting led to inflammation of tumor tissue and increased infiltration by cytotoxic CD8 T cells and natural killer cells. But it only reduced the rate of tumor growth in mouse models of melanoma and colon cancer because the activity of those immune cells was still limited by the immune checkpoint protein PD-1. However, blocking the activity of PD-1 with antibodies led to elimination of tumors that had been inflamed by anti-CBM treatment.
"Treg cells are preferentially 'auto-reactive,' meaning they react to our own, 'self' tissue antigens," explains Mempel, an associate professor of Medicine at Harvard Medical School. "By reprogramming Treg cells in tumor tissue, we create a local inflammatory autoimmune reaction that primes tumors for immune therapies. So instead of trying to get rid of Treg cells, we now can use them as an asset, harnessing their self-reactivity for cancer treatment."
Mauro Di Pilato, Ph.D., a research fellow in Dr. Mempel's lab and lead author of the study, adds, "Now we need to assess whether this approach works as well in humans as it does in mice and understand why Treg cells in the tumor environment, but not elsewhere, are reprogrammed through targeting of the CBM complex. The ability to reprogram Treg cells to improve patient response to immune checkpoint blockade has the potential of increasing the number of patients who can be helped with that approach."
More information: Targeting the CBM complex causes Treg cells to prime tumours for immune checkpoint therapy, Nature (2019). DOI: 10.1038/s41586-019-1215-2 , www.nature.com/articles/s41586-019-1215-2