Brighter possibilities for treating blindness

Advances in preclinical research are now being translated into innovative clinical solutions for blindness, a review published in the 10th Anniversary Series of Science Translational Medicine reports.
Gene replacement or gene editing strategies could potentially reverse vision loss and lead to close to normal visual outcomes. Early intervention during the early stages of retinal degeneration, when the photoreceptor cells (rods and cones) are still intact, is particularly promising. The first approved gene therapy for Leber congenital amaurosis (LCA) with confirmed biallelic RPE65 mutation has paved the way for more than 30 gene replacement trials worldwide in other conditions, e.g. those in clinical phase III for choroideremia, achromatopsia, and Leber hereditary optic neuropathy.
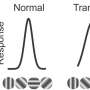
Gene-independent strategies aim to prevent or slow the progressive degeneration of photoreceptor cells with neuroprotective agents for a broad spectrum of retinal dystrophies. Neuroprotective strategies, particularly those for preserving cones, are the best approach for treating disease where there is ongoing photoreceptor cell degeneration.
Stem cell therapy, optogenetic therapy, and retinal prostheses are used to restore vision during the later stages of retinal degeneration. These approaches can be applied independently of the causal mutation and are expected to restore a low degree of vision in blind patients. Stem cell therapies to replace degenerated cells for restoring vision are under development or clinical evaluation in a wide range of retinal degenerative conditions, e.g. for the "non-neovascular" form (associated with gradual loss of photoreceptors and RPE cells) of age-related macular degeneration (AMD), for inherited retinal dystrophies (IRDs) and retinal pigment epithelium (RPE) replacement.
Brain-machine interface technologies using electrode arrays or optogenetics can stimulate the visual pathway downstream of the retina. Electrical stimulation of the primary visual cortex is one possible scenario that is currently in clinical trials.
Optogenetic therapy makes cells light sensitive through expression of an optogene encoding a light-activated channel or pump in the remaining inner retinal cells. It could be used to resensitize a degenerated retina to visible light independent of the mutation causing photoreceptor cell loss.
Retinal prostheses are able to reactivate remaining retinal circuits at the level of bipolar or ganglion cells after photoreceptor cell loss. Both epiretinal and subretinal implants are able to stimulate a light-insensitive degenerated retina and to restore partial vision in blind people.
Accelerating therapeutic developments in ophthalmology
Specific characteristics make the eye particularly suited for diagnostic and therapeutic exploration: easy access, small volume, high internal compartmentalization, and stable cell populations. The optical transparency of the eye allows direct visualization with high-resolution imaging and precise evaluation of disease stage and response to therapy. The relative immune privilege of the eye, especially the subretinal space, reduces adverse responses to injected vectors and gene products. However, progress in ophthalmology is intrinsically linked to increased understanding of the morphology and function of the visual system.
More information: José-Alain Sahel et al. Depicting brighter possibilities for treating blindness, Science Translational Medicine (2019). DOI: 10.1126/scitranslmed.aax2324