Stopping Parkinson's disease before it starts
An Osaka University-led research team has recently published findings that provide a ray of hope for the millions of Parkinson's disease (PD) sufferers worldwide. Although more common in those aged over sixty, PD can strike at any age, with an estimated prevalence of 41 per 100,000 people in their forties. And while not fatal in and of itself, the progressive neurodegeneration that is characteristic of PD can often cause secondary effects that lead to death.
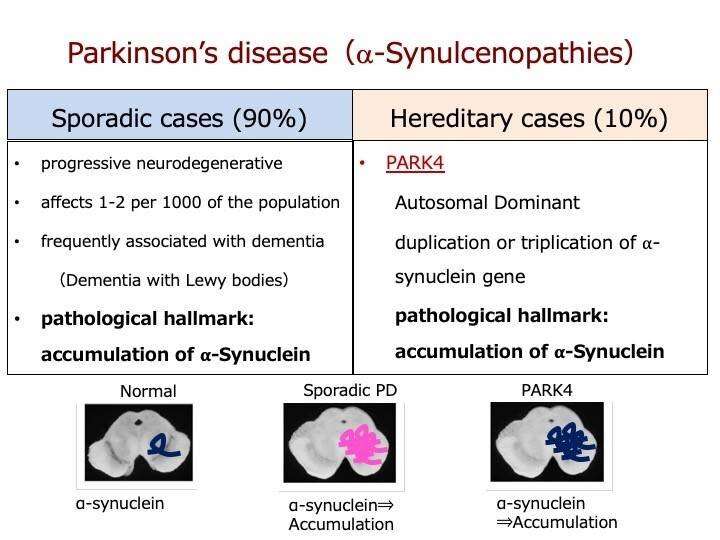
The exact cause of PD is still a mystery, but researchers believe that both genetics and the environment are likely to play a part. Importantly though, all PD patients show a loss of dopaminergic neurons in the brain and increased levels of a protein called α-synuclein, which accumulates in Lewy bodies. Lewy bodies are a pathological feature of both familial and sporadic forms of the disease, as well as some types of dementia.
In the study published this month in Scientific Reports, the team led by researchers from Osaka University's Graduate School of Medicine focused on α-synuclein as a target for a novel PD treatment.
"Although there are drugs that treat the symptoms associated with PD, there is no fundamental treatment to control the onset and progression of the disease," explains lead author Takuya Uehara. "Therefore, we looked at ways to prevent the expression of α-synuclein and effectively eliminate the physiological cause of PD."
To do this, the researchers designed short fragments of DNA that are mirror images of sections of the α-synuclein gene product. The constructs were stabilized by the addition of amido-bridging. The resulting fragments, called amido-bridged nucleic acid-modified antisense oligonucleotides (ASOs), bind to their matching mRNA sequence, preventing it from being translated into protein. After screening 50 different ASOs, the researchers settled on a 15-nucleotide sequence that decreased α-synuclein mRNA levels by 81%.
"When we tested the ASO in a mouse model of PD, we found that it was delivered to the brain without the need for chemical carriers," says co-lead author Chi-Jing Choong. "Further testing showed that the ASO effectively decreased α-synuclein production in the mice and significantly reduced the severity of disease symptoms within 27 days of administration."
Explains senior author of the study Hideki Mochizuki, "Our results showed that gene therapy using α-synuclein-targeting ASOs is a promising strategy for the control and prevention of PD. We expect that in the future, this method will be used to not only successfully treat PD, but also dementia caused by α-synuclein accumulation."
More information: Takuya Uehara et al. Amido-bridged nucleic acid (AmNA)-modified antisense oligonucleotides targeting α-synuclein as a novel therapy for Parkinson's disease, Scientific Reports (2019). DOI: 10.1038/s41598-019-43772-9