August 23, 2019 feature
Engineering collagen-binding serum albumin (CBD-SA) as a drug conjugate carrier for cancer therapy
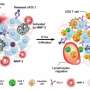
![Synthesis and characterization of Dox-CBD-SA. (A) Schematic of CBD-SA mediated drug delivery. (B) Synthesis scheme of Dox-CBD-SA. (C) Affinities [dissociation constant (Kd) values are shown] of CBD-SA and SA against collagen type I and collagen type III were measured by enzyme-linked immunosorbent assay (ELISA). N.D., not determined due to low signal. (D) Dox conjugation ratio per protein is presented. Values were calculated on the basis of the results of bicinchoninic acid assay protein quantification assay (proteins) and absorbance at 495 nm (Dox) (mean ± SD of three experimental replicates). (E) Dox release kinetics from Dox-CBD-SA under three different pH conditions was evaluated by fluorescence (excitation at 495 nm, emission at 590 nm) (n = 3, mean ± SD; two experimental replicates). (F) MMTV-PyMT cells were seeded and incubated overnight. Dox, Dox-SA, or Dox-CBD-SA was added (red). Cells were also stained with LysoTracker (green). Scale bars, 20 μm. Representative pictures are presented. Two experimental replicates. (G and H) Cytotoxicity of Dox variants against MMTV-PyMT cells or MC38 cells in vitro (n = 6, mean ± SEM). Credit: Science Advances, doi: 10.1126/sciadv.aaw6081 Engineering collagen-binding serum albumin (CBD-SA) as a drug conjugate carrier for cancer therapy](https://scx1.b-cdn.net/csz/news/800a/2019/1-engineeringc.jpg)
Medical researchers often use serum albumin (SA) as a drug carrier to deliver cytotoxic agents to tumors during biomedical drug delivery via passive targeting approaches. To improve the targeting capacity of SA's a team of scientists recently developed an approach to retain SA-drug conjugates in tumors by combining passive and active targeting mechanisms. In the new study, Koichi Sasaki and colleagues in the Pritzker School of Molecular Engineering in the University of Chicago, U.S., recombinantly fused SA with a collagen-binding domain (CBD) of the von Willebrand factor protein. The approach allowed binding within the tumor stroma after drug release, due to tumor-vascular permeability. The work is now published on Science Advances.
The research team then conjugated doxorubicin (Dox; an anticancer drug) to the CBD-SA conjugate using a pH-sensitive linker. The Dox-CBD-SA treatment significantly suppressed tumor growth compared to Dox-SA and aldoxorubicin treatment in a mouse model of breast cancer. Notably, the new compound—Dox-CBD-SA, efficiently stimulated host anti-tumor immunity to completely eradicate established tumors in an animal model of MC38 colon carcinoma when the research team combined the compound with the anti-PD1 checkpoint inhibitor. The compound DOX-CBD-SA decreased adverse side-effects compared to aldoxorubicin, confirming the bioengineered CBD-SA as a versatile, clinically relevant drug conjugate carrier protein with potential to treat solid tumors.
Serum albumin is the most abundant protein in blood and a number of small molecule compounds can be fused, conjugated with, or co-formulated with SA for improved drug delivery to disease lesions. The exceptionally long plasma half-life and hydrophilicity (water loving nature) of SA contributed to improve the pharmacokinetics, safety and efficacy of the new drug compounds. Notably, SA can passively target tumors via pathological permeability of the tumor vasculature, which is advantageous for cancer therapy. Molecular engineering approaches have gained prominence in the field of cancer research to combine passive and active targeting during tumor drug delivery.
Sasaki and team had previously shown the targeted delivery of checkpoint inhibitor (CPI) antibodies and the cytokine interleukin-2 (IL-2) using a collagen binding domain (CBD) known as the A3 domain of von Willebrand factor (VWF). The A3 domain of VWF has the highest affinity to collagen types I and III proteins. This is since collagen is abnormally expressed and exposed to the bloodstream due to hyperpermeability of the tumor vasculature, forming a promising target for cancer drug delivery. The bioengineered forms in the preceding study showed significantly stronger anti-tumor effects compared to their unmodified forms in mouse models of cancer. Researchers had also suppressed treatment-related adverse events by fusing CBD to the drug during the tumor targeting strategy. In the present work, Sasaki et al. hypothesized that CBD would be similarly compatible with SA-based drug delivery carriers to engineer an active serum albumin (SA) with tumor targeting potential.
The research team focused on Doxorubicin (Dox), a small-molecule anticancer drug approved by the US Food and Drug Administration (FDA) to treat a broad spectrum of cancer, where the molecule can internalize in proliferating cells through passive transmembrane diffusion to interfere with DNA functions and cause cell death. However, its antitumor efficacy is not notable due to acquired drug resistance, bone marrow suppression and excessive inflammation among patient populations, leading to a poor therapeutic index. To improve Dox efficacy, researchers often combine other chemotherapeutic agents, such as synergized treatment with CPI (checkpoint inhibitors) or Dox conjugated with SA for its release in the low pH (reportedly pH 6.5) tumor microenvironment in a mouse model.
In the present work, Sasaki et al. designed a recombinant mouse SA by fusing the collagen binding domain of the VWF A3 domain (CBD-SA). Then they conjugated aldoxorubicin (derivative of Dox) to CBD-SA using a pH-dependent cleavable hydrazone link, prior to experimental injection as the "Dox-CBD-SA" therapeutic agent. The research team tested the engineered CBD-SA as a tumor-targeting drug carrier for improved antitumor efficacy with Dox, in the tumor microenvironment of a translational animal model.
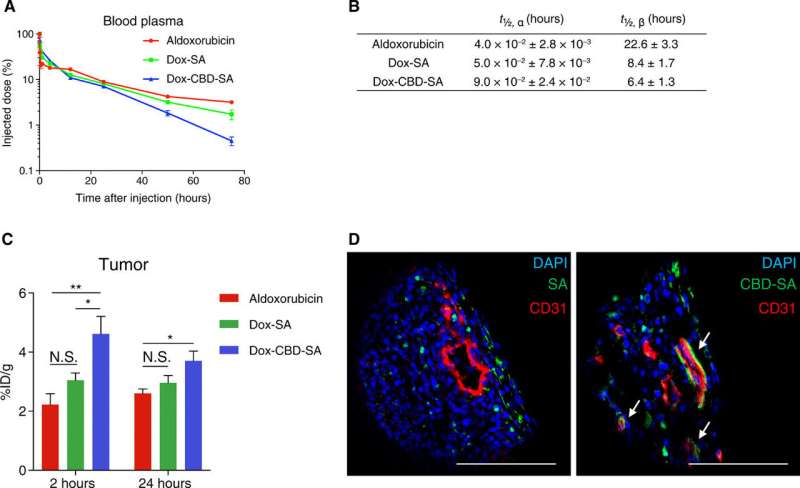
The scientists first synthesized the new drug conjugates to target the tumor microenvironment and investigated the binding potential of CBD-SA to the recombinant collagen protein in vitro to show strong binding affinities to collagen types I and III. They covalently conjugated aldoxorubicin to CBD-SA and conducted SDS-polyacrylamide gel electrophoresis (SDS-PAGE) to observe the monomeric structure of purified DOX-SA and DOX-CBD-SA molecules. They examined the release kinetics of Dox from conjugates under varying pH conditions to show maximum release at pH 5.0 and 6.5, consistent with previous reports on small chemical release kinetics in tumor microenvironments.
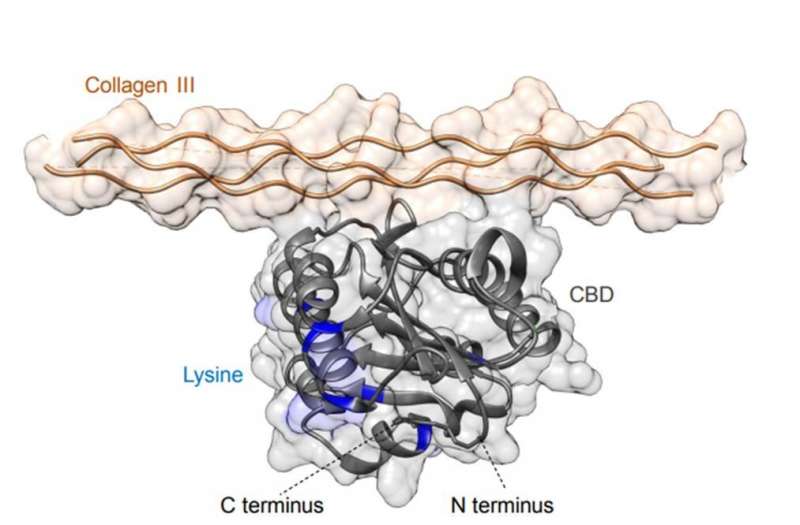
![Dox-CBD-SA shows enhanced antitumor efficacy and infiltration of lymphocytes into tumor in the MMTV-PyMT breast cancer model. (A) MMTV-PyMT cells (5 × 105) were inoculated into FVB mice on day 0. Aldoxorubicin, Dox-SA, or Dox-CBD-SA (5 mg/kg on a Dox basis) was injected intravenously on day 7. Graphs depict tumor volume until the first mouse died (mean ± SEM). (B) Survival rate. (C to F) Individual tumor growth curves. CR indicates complete response frequency. Three experimental replicates. (G to L) MMTV-PyMT cells (5 × 105) were inoculated on day 0. Aldoxorubicin, Dox-SA, or Dox-CBD-SA (5 mg/kg on a Dox basis) was injected intravenously on day 7. Lymphocytes within tumors were extracted on day 14, followed by flow cytometric analysis. (G to I) Graphs depict the number of (G) CD45+CD8+CD3+ T cells, (H) CD45+CD4+CD3+ T cells, and (I) CD45+NK1.1+CD3− NK cells per tumor weight (in milligrams). Bars represent mean ± SEM. (J to L) Graph shows [CD45+CD8+CD3+ T cells per tumor weight (mg)] (J), [CD45+CD4+CD3+ T cells per tumor weight (mg)] (K), or [CD45+NK1.1+CD3− NK cells per tumor weight (mg)] (L) versus [tumor weight]. Two experimental replicates. Statistical analyses were done using (A, H, and I) ANOVA with Tukey’s test or (G) Kruskal-Wallis test followed by Dunn’s test or (B) log-rank (Mantel-Cox) test. *P < 0.05; **P < 0.01. Credit: Science Advances, doi: 10.1126/sciadv.aaw6081 Engineering collagen-binding serum albumin (CBD-SA) as a drug conjugate carrier for cancer therapy](https://scx1.b-cdn.net/csz/news/800a/2019/4-engineeringc.jpg)
Using cell culture studies the team detected the presence of Dox in the cytoplasm of MMTV-PyMT cells (mouse mammary tumor virus-polyomavirus middle T antigen) after 1-hour of incubation. After 24-hours of incubation with Dox-conjugates they noted the uptake of the liberated drug due to the acidic pH within the intracellular organelles of the cancer cell line. Cell viability tests in the lab verified all forms of Dox in the study to have comparable cytotoxicity to cause cancer cell death in vitro.
Inspired by in-lab pharmacokinetics and tumor accumulation studies, the research team tested the anti-tumor effects of Dox-CBD-SA in vivo in tumor-bearing mice. For this, they injected the MMTV-PyMT orthotopic tumor-bearing mice with single intravenous injections of several Dox forms via the tail vein. The work showed that pre-conjugation of Dox with SA provided a higher therapeutic effect than the conjugation of aldoxorubicin with endogenous SA in situ. Importantly, Dox-CBD-SA showed greater therapeutic potential compared to Dox-SA by extending the survival rate and inducing tumor remission in 2 of 12 mice. The data supported the superiority of the Dox carrier compared to the unmodified SA, relative to antitumor efficacy.
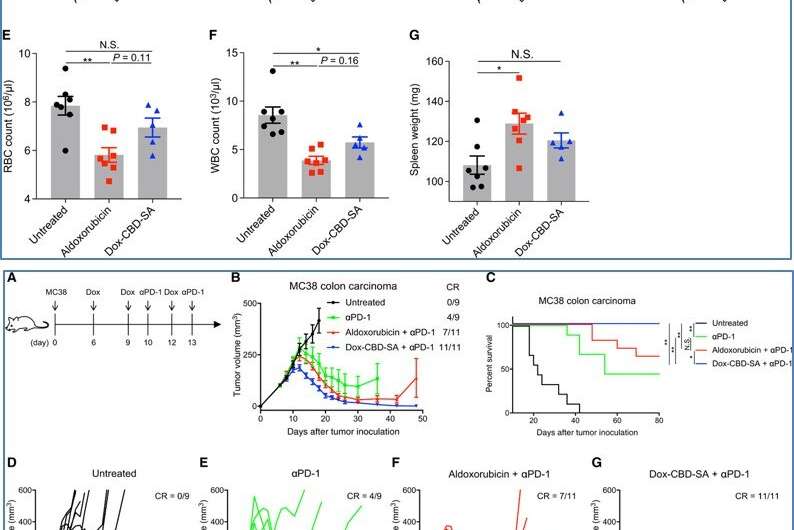
Dox also induced immunogenic cell death (ICD) to stimulate tumor-infiltrating lymphocytes (TIL), which were biomarkers for favorable prognosis in multiple cancers. Sasaki et al. therefore analyzed TILs after treatment with the new drug in the present work and recorded enhanced filtration of lymphocytes for antitumor effects. The researchers observed lower toxicity of Dox-CBD-SA compared to aldoxorubicin due to its slow release under physiological pH. For instance, aldoxorubicin increased the plasma concentration of inflammatory cytokines and decreased the red blood cell counts, white blood cell counts and hemoglobin concentration. By contrast, the adverse impact of Dox-CBD-SA was mild. The work suggested a reduced toxicity spectrum during treatment after pre-conjugation of Dox with CBD-SA.
The research team further investigated if combining Dox-CBD-SA with CPI (checkpoint inhibitors) showed greater therapeutic effects compared to aldoxorubicin therapy with CPI. For this, they used the MC38 colon carcinoma model, which was immunogenic but not curable with Dox monotherapy alone. They noted the new drug induced immunogenic cell death synergistically with the clinical CPI to achieve superior antitumor effects compared to aldoxorubicin and a clinical CPI.
In this way, Koichi Sasaki and colleagues developed a new therapeutic agent Dox-CBD-SA, which accumulated in tumors to activate host antitumor immunity. Monotherapy of the new agent suppressed orthotropic MMTV-PyMT breast tumor growth in an animal model and prolonged their survival. When combined with immune checkpoint inhibition, the drug completely eradicated tumors in an immunogenic MC38 mouse model. They conclude that the pre-conjugation of CBD-SA may hold potential for clinical translation during cancer therapy as an antitumor drug carrier
More information: Koichi Sasaki et al. Engineered collagen-binding serum albumin as a drug conjugate carrier for cancer therapy, Science Advances (2019). DOI: 10.1126/sciadv.aaw6081
Jun Ishihara et al. Targeted antibody and cytokine cancer immunotherapies through collagen affinity, Science Translational Medicine (2019). DOI: 10.1126/scitranslmed.aau3259
Robert C. Young et al. The Anthracycline Antineoplastic Drugs, New England Journal of Medicine (2010). DOI: 10.1056/NEJM198107163050305
© 2019 Science X Network