Restoring a gene altered in Down syndrome rescues adult neurogenesis and learning and memory defects in mice
A recent study, affiliated with South Korea's Ulsan National Institute of Science and Technology (UNIST) has discovered that restoring a gene altered in Down syndrome called the Down syndrome critical region 1 (DSCR1) rescued adult neurogenesis and learning and memory defects in a Down syndrome mouse model (Ts65Dn).
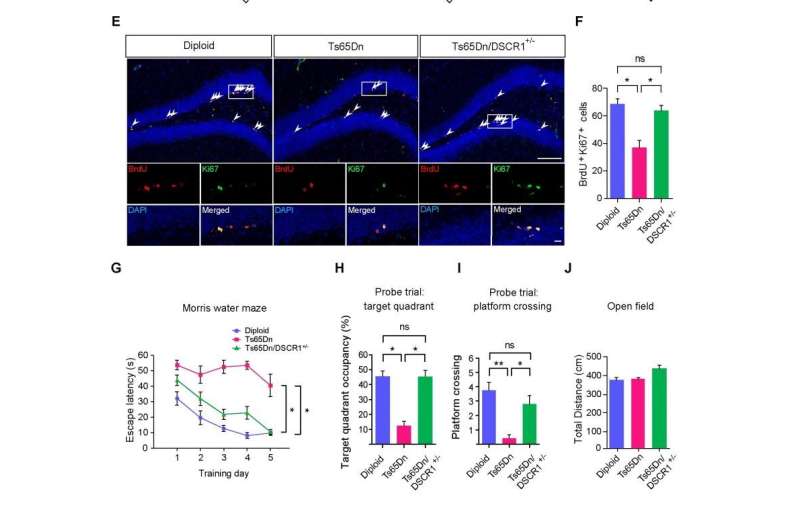
Professor Kyung-Tai Min and his research team in the School of Life Sciences at UNIST, Korea discovered that restoring a gene altered in Down syndrome called the Down syndrome critical region 1 (DSCR1) rescued adult neurogenesis and learning and memory defects in a Down syndrome mouse model (Ts65Dn). These results highlight that DSCR1 contributes to intellectual disability in Down syndrome, and further pave the way for treatment.
Adult neurogenesis is the process of generating new neurons in the adult brain. Defects in adult hippocampal neurogenesis have been observed in various animal models of neurological disorders including schizophrenia, depression, Parkinson's disease, Alzheimer's disease, and neurodevelopmental disorders such as Down syndrome. However, the precise cellular and molecular mechanisms underlying adult hippocampal neurogenesis and their links to neurological disorders are not well understood.
Min's team discovered that DSCR1 is required for adult hippocampal neurogenesis, and furthermore elucidated mechanisms underlying how DSCR1 regulates this process. They showed that DSCR1 binds to and modulates TET1 splicing, which subsequently controls miRNA-124 expression by regulating the methylation status of the miRNA-124 promoter. Loss of DSCR1 leads to increase TET1 levels, resulting in miRNA-124 promoter hypomethylation and increased miRNA-124 expression. DSCR1 transgenic mice display opposite changes, albeit they also have defects in adult hippocampal neurogenesis.
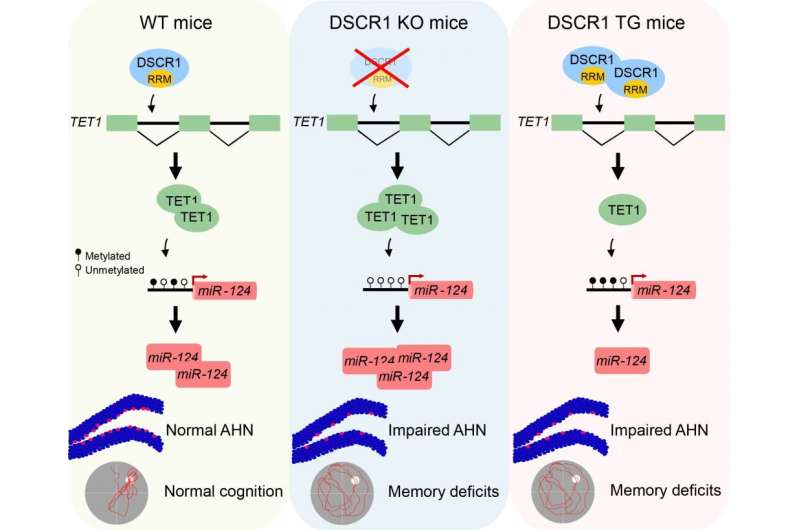
Strikingly, correcting DSCR1 dosage alleviates both the impaired adult hippocampal neurogenesis and the defective learning and memory seen in a Down syndrome mouse model (Ts65Dn). Together, the results reveal that precise regulation of DSCR1 and the interplay between TET1 and miRNA-124 are crucial for normal adult hippocampal neurogenesis. These findings further highlight potential therapeutic targets for the treatment of Down syndrome and other neurological disorders associated with adult neurogenesis.
The first author Chiyeol Choi, a Ph.D. student, explained "The expression of two epigenetic regulators (TET1 protein and miR-124), mediated by the DSCR1 protein, is important for regulating adult neurogenesis in the hippocampus. This is likely a key mechanism contributing to defective adult neurogenesis observed in the Down syndrome mouse model."
Professor Min noted "Our findings not only provide a basic understanding of the mechanisms regulating adult hippocampal neurogenesis, but will also contribute to the development of a novel therapy for the cognitive deficits manifested in Down syndrome patients."
More information: Chiyeol Choi et al. DSCR1‐mediated TET1 splicing regulates miR‐124 expression to control adult hippocampal neurogenesis, The EMBO Journal (2019). DOI: 10.15252/embj.2018101293