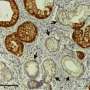
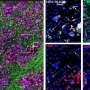
Revealing the molecular engine that drives pancreatic cancer provides ways to turn it off
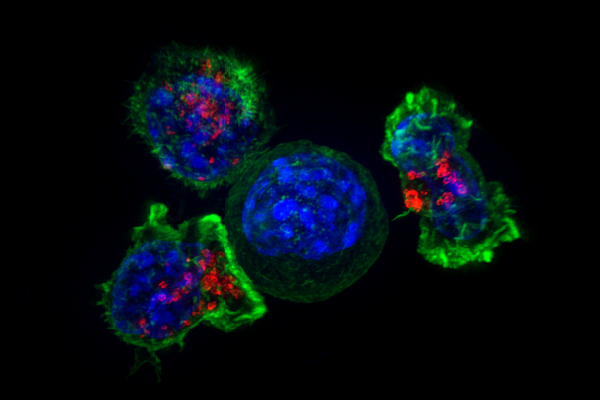
Researchers at Georgetown Lombardi Comprehensive Cancer Center have decoded a chain of molecules that are critical for the growth and survival of pancreatic ductal adenocarcinoma (PDAC)—the most common and also the most lethal form of pancreatic cancer.
They say their findings, published in Developmental Cell, suggest that inhibiting this "Yap" biological network may effectively regress early stage PDAC and could be paired with other drugs to halt more advanced stage tumors. Yap inhibitors have been developed and are moving into clinical trials.
Their study builds upon Georgetown Lombardi research that previously identified Yap as an oncogene central to the initiation of PDAC as well as a variety of other cancers. In the current study employing advanced animal models, they have managed to switch off Yap in pre-established PDAC tumors, and discovered that suppressing Yap blocks the metabolic pathways that provide the fuel and building materials for maintaining the growth of the cancer.
This study revealed the "flow chart" of key molecules in the Yap signaling network, which could be used to design novel and more effective therapies for advanced pancreatic cancer, says the study's senior investigator, Chunling Yi, Ph.D., associate professor of oncology at Georgetown Lombardi.
"Our research suggests that inhibiting Yap as well as Sox2, a molecule that gets turned on when Yap is inhibited, could be very important to long-term control of pancreatic cancer," says Yi. "In later stages of this cancer, when a Yap inhibitor is used, Sox2 could takes its place to allow PDAC to survive and grow, so therapy that targets both molecules would be ideal."
Five-year survival for PDAC is in the single digits because 80 percent of patients are diagnosed with late-stage disease. Although the disease is the 12th most common cancer in the U.S., it is the 4th leading cause of cancer death, according to the National Cancer Institute.
The vast majority PDAC (95%) is caused by a mutation in an oncogene known as Kras, which keeps cell growth switched on. There is no approved treatment for tumors with Kras mutations, which are found in a number of cancers.
Kras mutations activate the Yap pathway. Yi and her colleagues, which include investigators from Germany and France, show, in animal models, that the Yap protein is required for the maintenance of Kras mutant PDAC tumors.
In preclinical work, Yi demonstrated that inhibiting Yap can force PDAC tumors to regress into cells that resemble what are normally found within the organ. Eventually, however, Sox2 is activated to compensate for loss of Yap, causing some tumors to relapse.
"To effectively control tumor growth, you have to know the molecular network that drives that growth. This study takes a good look under the hood and gives us the key drivers that could be targeted to shut that engine down," Yi says.
More information: Developmental Cell (2019). DOI: 10.1016/j.devcel.2019.07.022 , www.cell.com/developmental-cel … 1534-5807(19)30628-8