Breakthrough in harnessing the power of biological catalysts

The power of nature could soon be used to create day-to-day materials such as paints, cosmetics and pharmaceuticals in a much more environmentally friendly way, thanks to a new breakthrough from scientists.
The international team, involving Dr. Simon Freakley from the Centre for Sustainable Chemical Technologies at the University of Bath, has successfully unlocked the catalytic abilities of enzymes taken from fungi by creating the perfect conditions needed for them to function.
This could potentially lead to greener ways of creating a host of industrial chemicals in a much more efficient way, by combining the enzyme with a heterogeneous catalyst and producing only water as the reaction by-product.
Catalysis is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst.
Catalysts are widely used in industry to produce products in a much quicker and more efficient way, with the global catalysis market valued at more than $25 billion.
Yet scientists are constantly on the look-out for potential new catalysts and often look to nature for inspiration. Enzymes, which are known to catalyze numerous biochemical reaction types, are unrivaled when it comes to speeding up chemical reactions at mild conditions and have long been obvious candidates.
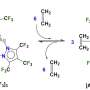
Of particular interest to scientists are enzymes known as peroxygenases which are derived from fungi, among other organisms.
To function effectively when used in industry, enzymes need a steady supply of oxidant, which for peroxygenases is usually provided from hydrogen peroxide (H2O2).
The H2O2 itself is often provided by another supporting catalyst, with current approaches using additional enzyme systems, but this often results in complicated reaction mixtures.
A new approach has been to combine hydrogen (H2) and oxygen (O2) directly to produce the H2O2; however, the specific catalysts used for this type of reaction work at very harsh conditions that enzymes do not like.
As such, this has provided a major hurdle for scientists trying to maximize the catalytic potential of enzymes as they have found it difficult to develop supporting catalysts that can operate in an enzyme's ideal environment without causing damage to the enzyme itself.
In their new study, published in the journal Nature Communications, the team has successfully developed a catalyst made from gold and palladium nanoparticles that can produce a steady stream of H2O2 to the enzyme in much more benign conditions. This is consumed by the enzyme in the same reaction vessel to carry out the chemical transformation, resulting in only water as a by-product of the whole combined catalytic process.
Lead author Dr. Freakley said: "Our catalyst can produce just the right amount of H2O2 for the enzyme to drive the whole process at mild conditions. These transformations would require much harsher conditions if using just traditional heterogeneous catalysts.
"We show that the H2O2 is consumed by the enzyme to oxidize a range of organic molecules with high selectivity.
"This is an extremely significant step towards utilizing the power of enzymes to create a range of molecules, from commodity to fine chemicals, in a much greener and more efficient way. Showing the possibility that biocatalysts can be integrated into the current chemical infrastructure."
More information: Simon J. Freakley et al. A chemo-enzymatic oxidation cascade to activate C–H bonds with in situ generated H2O2, Nature Communications (2019). DOI: 10.1038/s41467-019-12120-w
Journal information: Nature Communications
Provided by University of Bath