Inflammation amps up neurite growth, gene expression involved in heat, cold sensitivity
Researchers from North Carolina State University have found that inflammation increases neuronal activity, gene expression and sensory nerve (neurite) outgrowth in neurons involved in thermal—but not physical- sensations in mice. The work sheds light on the role that inflammation-induced overexpression of calcium channel genes may play in pain hypersensitivity.
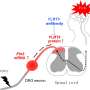
Inflammation can often cause pain hypersensitivity due to a number of factors: increased expression of pain receptors; altered neurotransmitter release in the spinal cord; heightened excitability of neurons; and—as demonstrated in these new findings—physical changes such as the growth of more neurites (sensory nerve projections in neurons). Voltage-gated calcium channels (VGCCs) play an important role in all of these changes, as the neurotransmitters they release control neuron-to-neuron communication.
"In inflammatory states, VGCCs play a role in sensory neurons becoming overactive, or hyperexcited." says Santosh Mishra, assistant professor of molecular biomedical sciences at NC State and lead author of a paper describing the work. "Additionally, the calcium molecules released and controlled by these channels regulate neurite growth. We wanted to look more closely at the role of a VGCC called Cav2.2, to see if it increased peripheral neurite outgrowth during inflammation."
In the peripheral nervous system, neurons are tuned to produce specific nociceptive signals. The TRPV1 and TRPM8 sensory neurons, for example, are associated with thermal sensations like heat and cold. MrgprD- and MrgprB4-expressing neurons, on the other hand, are associated with potentially damaging (like pinching) and low threshold mechanical sensations (like pleasant touch) respectively.
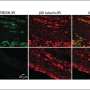
Mishra's team chose Cav2.2 due to its abundance in the dorsal root ganglia (DRG), which are clusters of sensory cells located at the root of the spinal nerves. Using an inflammatory mouse model with both in vitro and in vivo approaches, the team looked at the relationship between inflammation, Cav2.2 activity and afferent neurite growth (afferent neurons in the peripheral nervous system carry stimuli to the central nervous system). They found that inflammation increased expression of Cav2.2, which in turn increased afferent neurite growth and activity in thermosensitive neurons, but not in mechanical neurons.
"There was no increased expression of Cav2.2 channels in the mechanical neurons, and so there was no discernable effect on these neurons," Mishra says. "What we don't understand is why inflammation doesn't induce upregulation of calcium channels in mechanosensation the way that it does with thermal sensation. It could be that those neurons don't express as much of the Cav2.2 VGCC to begin with, but it is something that we will have to investigate further."
Mishra hopes that the work will help scientists uncover more about causes of chronic pain—particularly whether changes in peripheral nerve growth due to inflammation play a role in the shift from acute to chronic pain states.
"Most pain research focuses on particular receptors and neurotransmitters, not the actual sensory network such as afferent growth," Mishra says. "Afferents are like antennae. A receptor will tell you if the connection between the signal and the carrier is good, but antennae carry the receptors—so if there are more antennae, you receive more signal. In this model inflammation created more thermosensitive antennae, and so those sensations were felt more acutely."
The work appears in Frontiers in Neuroscience.
More information: Saumitra Pitake et al, Inflammation Induced Sensory Nerve Growth and Pain Hypersensitivity Requires the N-Type Calcium Channel Cav2.2, Frontiers in Neuroscience (2019). DOI: 10.3389/fnins.2019.01009