Stem cell companies using new US laws to provide unapproved products to seriously ill patients
A new US law, designed to give terminally ill patients access to unproven drugs, is being invoked by stem cell companies to make unapproved stem cell products available, in a move that has an Australian expert concerned.
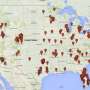
Professor John Rasko is one of a group of experts who are warning that the US Right to Try Act is being used by stem cell companies to provide unapproved products to seriously ill patients.
They say the use of this law by businesses selling unapproved stem cell products raises unsettling questions about their commitment to evidence based care.
They say the US is already home to hundreds of businesses selling unproven stem cell interventions, and the weakening of laws makes it even more difficult for regulators to ensure the safety of these products.
More information: Douglas Sipp et al. Stem Cell Businesses and Right to Try Laws, Cell Stem Cell (2019). DOI: 10.1016/j.stem.2019.08.012