X-ray experiments contribute to studies of a drug now approved to combat tuberculosis

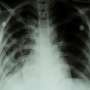
The U.S. Food and Drug Administration has approved a new antibiotic that, in combination with two existing antibiotics, can tackle one of the most formidable and deadly treatment-resistant forms of the bacterium that causes tuberculosis. The new antibiotic, called pretomanid (PA-824), can work with the other drugs like a deadly cocktail—triggering the bacteria (Mycobacterium tuberculosis) to release nitric oxide. This can burst the bacteria's cell walls and poison the microorganisms.
Studies exploring the structure and function of the new drug benefited from X-ray experiments at Berkeley Lab's Advanced Light Source (ALS). ALS experiments detailed the molecular structure of Ddn, a tuberculosis bacterium enzyme, in the presence and absence of a coenzyme (F420). Coenzymes, or cofactors, can help enzymes carry out chemical reactions. SLAC National Accelerator Laboratory's Stanford Synchrotron Radiation Lightsource (SSRL) also carried out related experiments.
Drug-resistant strains of tuberculosis bacteria infected an estimated 558,000 people in 2017. Existing treatments are often unsuccessful and can include as many as eight antibiotics taken for 18 months or longer. The World Health Organization has reported a 55 percent success rate in treating multi-drug resistant tuberculosis using existing treatments.
In a Phase III clinical trial, the three-drug regimen that includes the new FDA-approved antibiotic cleared the infection within six months from 95 of 109 patients who were unresponsive to previous treatments.
Provided by Lawrence Berkeley National Laboratory