AAV vector integration into CRISPR-induced DNA breaks
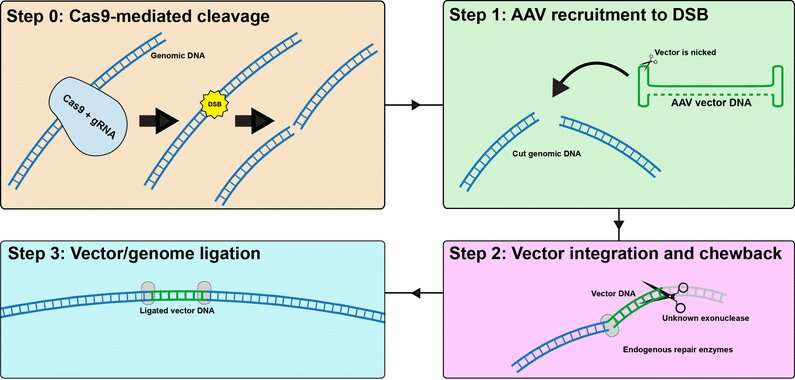
To design safe clinical trials, it is crucial to better understand and predict gene editing outcomes in preclinical studies. Bence György and collaborators have shown that adeno-associated viruses (AAVs) can stably integrate into CRISPR-Cas9-induced double-strand breaks, in up to almost half of the therapeutically targeted cells, in vitro and in vivo in mice. The team also showed that CRISPR did not cause an increase in genome-wide integration of AAV, but only at the CRISPR-cut site.
"This study helps to better understand the genomic consequences of therapeutic genome editing. It is currently not clear whether this site-specific AAV integration influences the safety profile of gene editing enzymes. We do not expect this, because as long as gRNAs are well-designed there is no increase of AAV integration at off-target sites. We will elaborate on these observations in further studies," says Bence György.
Widely used tools for genome editing and gene therapy
In the seven years since CRISPR was brought to the forefront of molecular biology, it has emerged as one of the most widely used (and hotly anticipated) tools for genome editing and gene therapy, with human trials now beginning.
In order to deliver this powerful molecular machine to target cells, many researchers use AAVs. This small virus has become the most widely used vector for delivering gene therapy, with three AAV-based therapies now on the market. It allows for the safe and stable expression of therapeutic genes in a wide array of cells types by delivering a small payload of DNA into cells' nuclei.
AAV vector integration after CRISPR intervention
AAV used for gene therapy lacks dedicated machinery to integrate into the genome. Therefore therapeutic AAV integrates at a very low frequency of <0.1%. In this study, the authors wondered whether this is also true for AAVs carrying genome-editing components.
In a multidisciplinary effort, the team (which also included Adrienn Volak, Alissa Muller and Stefan Spirig from IOB) collaborated with experts in the brain, inner ear and muscle to examine AAV integration in multiple organs after a CRISRP-mediated therapeutic intervention. Surprisingly, they found that AAV can stably integrate into on-target CRISPR-cut sites with up to 47% efficiency, in vitro and in vivo in mice. This means that almost half of the therapeutically targeted cells had this rather unexpected outcome.
"It took years for us to properly understand and characterize AAV integration. While analyzing sequencing results from mice injected with AAV vectors carrying CRISPR to disrupt an important dominant deafness gene, Tmc1, we have observed unique viral sequences in the target gene. This was surprising, as we really did not expect AAV genome to be there. Taken together, we have shown in this study that AAV integration is a gene editing outcome in vivo and it has to be considered when assessing safety," says Bence György, who directed the study during his postdoc at the Massachusetts General Hospital and Harvard Medical School.
No genome-wide integration of AAV, only at the CRISPR-cut site
The team also ran extensive analyses to determine if the presence of CRISPR caused an increase in genome-wide integration of AAV. Importantly, this was not found to be the case—AAV integration rates were only heightened at the CRISPR-cut site, and controls lacking a gRNA showed no integration increase over baseline. This suggests that CRISPR is highly specific to the gene it was designed to target.
"The results also suggest that AAV integration could be used as a sensitive assay to detect the presence of double-stranded breaks. In the future this might be applied to directly assaying genome-wide specificities of genome editing nucleases, as integrated AAV marks CRISPR target sites," says Bence György.
More information: Killian S. Hanlon et al. High levels of AAV vector integration into CRISPR-induced DNA breaks, Nature Communications (2019). DOI: 10.1038/s41467-019-12449-2
Journal information: Nature Communications
Provided by Institute of Molecular and Clinical Ophthalmology Basel