Study demonstrates HIV antibody responses within six weeks of initial vaccination
An early phase study was conducted in the U.S. in which different combinations of DNA (DNA-HIV-PT123) and protein (AIDSVAX B/E) vaccines were administered in four randomized treatment groups (T1, T2, T3, T4), to determine which strategy would induce favorable HIV-specific antibody and T-cell responses. The study led by protocol co-chair and Investigator at the Emory Center for AIDS Research Nadine Rouphael, M.D. and chair and professor in the Department of Medicine at the University of Rochester Medical Center Michael Keefer, M.D. demonstrated that DNA (DNA-HIV-PT123) and protein (AIDSVAX B/E) combination vaccine regimens induced high magnitude and long-lasting binding antibody responses and that more rapid potentially protective immune responses were observed when the vaccine regimens were co-administered. The "DNA priming and gp120 boosting induces HIV-specific antibodies in randomized clinical trial" findings were published in the Journal of Clinical Investigation on September 30.
HVTN 105 was conducted by the HIV Vaccine Trials Network (HVTN), headquartered at the Fred Hutchinson Cancer Research Center and funded by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health, and sponsored by NIAID.
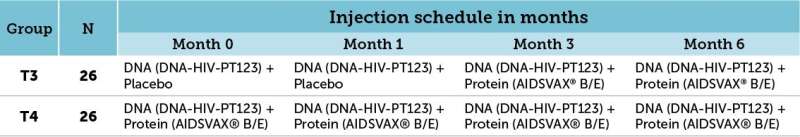
One-hundred and four study participants at low risk for HIV acquisition were enrolled in the study HVTN 105. HVTN 105 was designed to build upon the promising results from the U.S. Army-led RV144/Thai trial, which was the first vaccine clinical trial to ever demonstrate any efficacy in preventing HIV. Study participants were randomized to one of four treatment groups and received intramuscular injections at 0, 1, 3 and 6 months. All vaccine regimens were safe and well tolerated in all treatment groups. Of interest were the comparisons drawn between the vaccine-induced immune responses observed in the T3 and T4 treatment groups. Study participants in the T3 treatment group were vaccinated with a modified RV144 vaccine regimen where the ALVAC vector used in RV144 was substituted with DNA at months 0 and 1 and followed by DNA and protein co-administered at months 3 and 6. Study participants in treatment group T4 were vaccinated with DNA and protein co-administered at all four vaccination timepoints (0, 1, 3 and 6 months)
Because the partial efficacy of the RV144 vaccine regimen seemed to be due to several identified correlates of protection, the authors of the current study investigated whether those same responses were induced by HVTN 105 vaccine regimens. Similar to RV144, the T3 and T4 regimens induced antibodies that bound to gp120 (HIV Env) and the V1V2 region of Env in 95-100% of study participants. T-cell responses were detectable in all treatment groups in HVTN 105. Both T3 and T4 demonstrated high antibody-dependent cellular cytotoxicity (ADCC) and neutralizing antibody responses. The T4 regimen induced a lower IgA/IgG ratio than T3, suggesting an improvement over the RV144 regimen, as this indicates a decrease in this correlate of risk. An additional surprising finding in T4 was the high levels of IgG4 responses, the significance of which in the context of HIV infection is not known.
"Our study shows that there are tools available to us now to improve on the immunogenicity seen in RV144, which may lead to better efficacy in future field trials," said protocol Co-Chair and Investigator at the Emory Center for AIDS Research Nadine Rouphael, M.D.
Vaccine-induced antibody and T-cell responses, observed in an early-phase clinical trial such as HVTN 105, are important measures to help determine if a vaccine regimen has the potential to be tested as a candidate vaccine in a large-scale trial, powered to determine efficacy.
More information: Nadine G. Rouphael et al. DNA priming and gp120 boosting induces HIV-specific antibodies in a randomized clinical trial, Journal of Clinical Investigation (2019). DOI: 10.1172/JCI128699