Mechanism of beta-cells involved in the development of type-1 diabetes revealed

Researchers Lorenzo Pasquali and Mireia Ramos-Rodríguez have published recently in Nature Genetics the results of a study on the mechanisms that cause an inflammatory response to trigger pancreatic beta-cell death, leading to the onset of Type 1 Diabetes (T1D). Pasquali is a Ramon y Cajal researcher at the Josep Carreras Leukaemia Research Institute (IJC), affiliated with CIBERDEM and the Germans Trias i Pujol Research Institute (IGTP), where he leads the Endocrine Regulatory Genomics group. Mireia Ramos-Rodríguez, the first author of the paper, is a doctoral student in the Endocrine Regulatory Genomics group. The study was carried out in collaboration with Decío Eizirik of ULB (Brussels, Belgium).
In T1D, the immune system selectively destroys pancreatic beta cells, depriving this organ of the capacity to produce insulin, and thus control blood sugar. In the search for why some people develop T1D, researchers have identified more than 60 regions on the genome where there are genetic variants associated with a higher risk of developing T1D, but their functions are not known yet. Additionally, most of these variants are located in DNA sequences that don't encode proteins and are dismissed as "junk DNA."
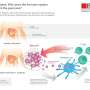
The immune attack against pancreatic beta cells is orchestrated by T and B cells, immune system cells. These cells infiltrate the pancreatic islets where beta cells live and, by releasing cytokines and chemokines, "dialogue" with the insulin-producing beta cells. Some misunderstanding in this "dialogue" is what ultimately causes beta cells to lose their functions and die. To test the mechanisms underlying this initial stage of the disease, the group analysed changes in gene expression, protein production, and DNA structure in beta-cells exposed to inflammatory cytokines.
Only about two percent of the genome contains sequences that correspond to genes that will translate into vital proteins. Conversely, much larger parts of the DNA that did not code for genes were thought to have no function. Scientists are increasingly discovering that these regions are significant and rich in regulatory sequences that act as "switches" and control which genes are turned on and off.
The group found that exposure to inflammatory cytokines changes the regulation of genes in the beta-cells and affects their functioning. They have mapped approximately 3,600 regions in the non-coding DNA that activates in the pancreatic islets. They also observed that exposure to inflammatory cytokines induces changes in DNA folding, allowing these non-coding regions to come into contact with their target genes. As a result, thousands of genes are switched on and translated into proteins.
The team found that genetic variants related to an increased risk of developing T1D occur in these newly mapped regions of the genome. "DNA variants in such regulatory elements may affect the capacity of the insulin-producing cells to react to an inflammatory environment," says Dr. Pasquali, "this knowledge will allow us to understand the detailed mechanisms whereby specific DNA variants predispose to type 1 diabetes," he adds.
Pasquali's group has used a model of particular interest, as it mimics the conditions islet cells might experience in the early stages of T1D. Much of the T1D genetic risk was shown to affect the immune cells, enforcing their role in the development of the disease. The islet cells themselves have been shown to have genes that control the critical steps in responding to danger signals and the innate immune system. However, linking islet gene regulation to T1D risk has yielded no significant results, until now. This new data opens the door to uncovering molecular mechanisms acting in the islet cells of the pancreas.
Now that the group has mapped out the switches that activate genes responding to an inflammatory environment in the pancreatic islets, it will be much easier to test different hypotheses of how common genetic variants are affecting the islet cells in T1D. Future work could look at different ways that the immune system stresses beta cells in later stages of the disease, for example.
"These results could apply to other diseases," says Mireia Ramos-Rodríguez. "In many cases, we do not understand why the immune cells attack a certain cell type. The dissection of regulatory elements in disease-relevant stimuli brings us closer to understanding the molecular mechanisms not only of T1D but also other autoimmune diseases."
More information: Mireia Ramos-Rodríguez et al. The impact of proinflammatory cytokines on the β-cell regulatory landscape provides insights into the genetics of type 1 diabetes, Nature Genetics (2019). DOI: 10.1038/s41588-019-0524-6