Could resetting our internal clocks help control diabetes?
The circadian clock system (from the Latin "circa diem," about a day) allows organisms to anticipate periodic changes of geophysical time and to adjust to those changes. Nearly all cells comprise molecular clocks that regulate and synchronize metabolic functions to a 24-hour cycle of day-night changes. Today, increasing evidence show that disturbances in internal clocks stemming from frequent time zone changes, irregular work schedules and aging have a significant impact on the development of metabolic diseases in human beings, including type-2 diabetes.
Such disturbances seem to prevent the proper functioning of the cells in the pancreatic islet that secrete insulin and glucagon, the hormones that regulate blood sugar levels. By comparing the pancreatic cells of type 2 diabetic human donors with those of healthy people, researchers at the University of Geneva (UNIGE) and at the University Hospitals of Geneva (HUG), Switzerland, were able to demonstrate, for the first time, that the pancreatic islet cells derived from the Type 2 Diabetic human donors bear compromised circadian oscillators. The disruption of the circadian clocks was concomitant with the perturbation of hormone secretion. Moreover, using clock modulator molecule dubbed Nobiletin, extracted from lemon peel, the researchers succeeded in "repairing" the disrupted cellular clocks and in partial restoring of the islet cell function. These results, published in the Proceedings of the National Academy of Sciences, provide a first insight into innovative approach for diabetes care.
Two years ago, the team led by Charna Dibner, principal investigator at UNIGE Faculty of Medicine, has already shown that in rodents, the perturbation of pancreatic cellular clocks led to disrupted insulin and glucagon secretion, thus promoting the onset of diabetes. But what is the situation in human beings?
Volodymyr Petrenko, a researcher in Dr. Dibner's lab and the first author of these publications, says, "We had also previously observed that if the clocks of human pancreatic cells were artificially disrupted in the cellular culture in vitro, secretion of the key islet hormones—insulin and glucagon—was compromised. Hence, our next step, that we report here, was to unravel whether the circadian rhythms were perturbed in human pancreatic islets in type 2 diabetes, and, if so, how would this perturbation affect the islet function."
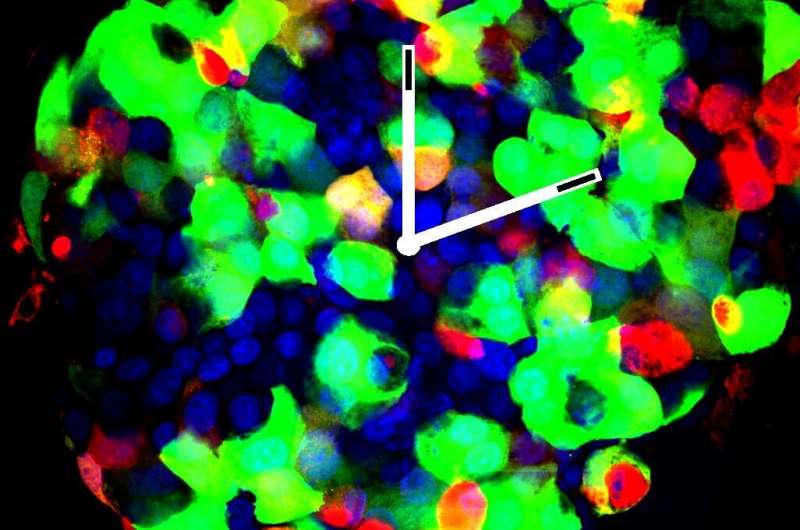
Using combined bioluminescence-fluorescence time-lapse microscopy, a technology that allows tracking the molecular clock activity in living cells very precisely over time, the scientists compared the behavior of pancreatic cell of type 2 diabetic donors and those of healthy subjects throughout the day. "The verdict is indisputable," says Charna Dibner. The biological rhythms of the islet cells in type-2 diabetes exhibit both reduced amplitudes of circadian oscillations and poor synchronization capacity. "As a result, hormone secretion is no longer coordinated. Moreover, the defects in temporal coordination of insulin and glucagon secretion observed in patients with type-2 diabetes were comparable to those measured in healthy islet cells with artificially-disrupted circadian clock."
It's all in the timing
Circadian clocks represent the daily cycles governing the various cellular functions. There are several interlocking levels of synchronization of these clocks, the main one being light, which in particular regulates the central clock located in the cerebral hypothalamus. Like a conductor in the orchestra, it regulates peripheral clocks present in organs and cells. The latter are therefore partly centrally regulated, but function differently in each organ, and even in each cell, depending on their functions. "Pancreatic cells are also subject to the rhythm of fasting and food intake, and to a tight hormonal regulation," says Charna Dibner. "Coordinating all levels of regulation therefore allows the optimization of metabolic functions. Clock deregulation in pancreatic islet leads to a compromised function: They stop anticipating food-derived signals. Indeed, if you eat the same food, but at night rather than during the day, you may gain weight much faster due to a suboptimal response of your metabolism."
Setting the right time again
Step two of their research: the Geneva scientists used Nobiletin, a small clock modulator molecule and a natural ingredient of lemon peel, in order to resynchronize the clocks. "By acting on one of the core-clock components, it efficiently resets the amplitude of the oscillations in the human islets" says Volodymyr Petrenko. "And as soon as we got the clocks back in sync, we also observed an improvement in insulin secretion."
"This is the first proof of principle that repairing compromised circadian clocks may help improve the function of pancreatic islet hormone secretion," says Charna Dibner. "We will continue by exploring this repair mechanism in vivo, first in animal models. Our society has experienced epidemic growth in metabolic diseases, concomitant with shifted working and eating schedules, and lack of sleep. By re-synchronizing the perturbed molecular clocks, either by personalized eating and exercise schedules or with the help of clock modulator molecules, we hope to ultimately be able to provide an innovative solution to an epidemic metabolic problem affecting an ever-increasing proportion of the world's population."
More information: Volodymyr Petrenko et al, In pancreatic islets from type 2 diabetes patients, the dampened circadian oscillators lead to reduced insulin and glucagon exocytosis, Proceedings of the National Academy of Sciences (2020). DOI: 10.1073/pnas.1916539117