New pieces added to the molecular puzzle of rheumatoid arthritis

Walter and Eliza Hall Institute researchers have revealed new details about how joint inflammation evolves in rheumatoid arthritis, and the cells that prolong the inflammatory attack.
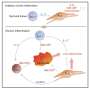
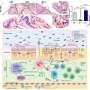
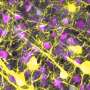
In both laboratory models and human clinical samples, the research team pinpointed immune cells called natural killer (NK) cells as an unexpected source of the inflammatory protein GM-CSF in rheumatoid arthritis, the first clue that these cells contribute to inflammatory autoimmune diseases. The research also explained how GM-CSF signals to other immune cells to prolong joint inflammation, and how GM-CSF signalling to immune cells is kept in check in healthy joints.
These discoveries could indicate potential new therapeutic targets for reducing joint inflammation in rheumatoid arthritis, and could potentially reduce inflammation in other autoimmune disease such as multiple sclerosis.
The research was published in the Journal of Experimental Medicine by a team co-led by Professor Ian Wicks, Professor Nicholas Huntington and Dr. Cynthia Louis, with Dr. Fernando Souza-Fonseca-Guimaraes.
At a glance
- The cell signalling protein GM-CSF causes inflammation that occurs in joints during rheumatoid arthritis.
- Our researchers have identified natural killer (NK) cells as a major source of GM-CSF in rheumatoid arthritis, the first time these cells have been implicated in an autoimmune disease.
- The team also identified the protein CIS as a key molecular brake that dampens GM-CSF activity and inflammation, revealing a potential new therapeutic avenue for inflammatory diseases.
A surprising source of GM-CSF
Rheumatoid arthritis is a chronic inflammatory autoimmune disease in which the immune system mistakenly attacks joints and other tissues, causing inflammation, pain and long-term joint damage.
GM-CSF was originally discovered at the Walter and Eliza Hall Institute as a growth factor for blood cells, but it is increasingly recognised as a key inflammatory mediator that drives a number of autoimmune diseases.
Professor Wicks said that his team's earlier research, together with colleagues at the University of Melbourne, had identified the signalling protein GM-CSF as an important contributor to joint inflammation in rheumatoid arthritis. "When we removed GM-CSF, we could see a reduction in inflammation. This finding underpinned the development and current clinical trials of inhibitors of GM-CSF signalling as a new approach to treating rheumatoid arthritis," he said.
"Although we knew that GM-CSF signalling was important in joint inflammation, which cells were producing GM-CSF within joints, and how this protein signalled after binding to its receptor on other immune cells, was not well understood."
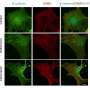
Dr. Louis said the team discovered that GM-CSF in inflamed arthritis joints was produced by immune cells called natural killer (NK) cells. "This was a surprise because, until now, NK cells were thought to primarily be important for clearing virus-infected or cancer cells," she said. "This is the first time NK cells have been found to contribute to tissue inflammation in autoimmune diseases such as rheumatoid arthritis.
"As well as looking at our laboratory model of arthritis, we examined cells from the joints of people with rheumatoid arthritis and confirmed that NK cells are indeed a significant source of GM-CSF in patients.
"This discovery has solved one part of the puzzle about how inflammation occurs in rheumatoid arthritis," Dr. Louis said.
Filling in the gaps
The team revealed that the protein CIS is important for 'switching off' GM-CSF signalling, a critical mechanism to restrain destructive inflammation in arthritis.
"In the absence of CIS, we saw hyperactivation of GM-CSF signalling and more severe arthritis," Dr. Louis said.
"This research showed that if a new drug that mimics CIS were to be developed, it may help to reduce the debilitating effects of GM-CSF in rheumatoid arthritis, but also in other inflammatory diseases driven by GM-CSF, such as multiple sclerosis."
Professor Wicks said the research revealed new aspects of cell signalling that warranted further investigation. "We're very excited to have progressed our understanding of rheumatoid arthritis and potentially other inflammatory diseases," he said.
More information: Cynthia Louis et al. NK cell–derived GM-CSF potentiates inflammatory arthritis and is negatively regulated by CIS, Journal of Experimental Medicine (2020). DOI: 10.1084/jem.20191421