Researchers shed light on anti-adhesive molecule in vascular endothelium
Researchers from the Harvard Medical School (HMS) Department of Ophthalmology and the Schepens Eye Research Institute of Massachusetts Eye and Ear have gained new insight into how a non-inflammatory state is maintained in the body. Their work focuses on the role of endomucin, a key molecule with anti-adhesive properties that encourages neutrophils - prevalent white blood cells that sense signals of injury—to travel past the vascular endothelium. Their findings, published in the current issue of Nature Communications, represent a paradigm shift in our understanding of inflammation.
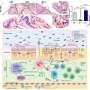
Blood cells move through the circulatory system from the heart through the arteries to the smallest capillaries, and then to the veins and back to the heart. The vascular system is lined with the endothelium, a thin layer of cells that serve as an interface between the blood and the tissues. When there is an injury or disease process, the body sends out signals that recruit circulating neutrophils to stick to the endothelium; the neutrophils then migrate between the endothelial cells to and pass into tissue. The accumulation of neutrophils and other white blood cells in the injured tissues is facilitated an increase in adhesive molecules on the surface of the small vessels in injured tissue.
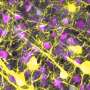
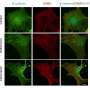
The research, led by Drs. Patricia D'Amore, Charles L. Schepens Professor of Ophthalmology and Pablo Argüeso, Associate Professor of Ophthalmology, both at Harvard Medical School, shows that in healthy, non-inflamed tissue, endomucin plays a critical role in preventing the neutrophils from sticking to the endothelium. During inflammatory conditions, however, the endomucin on the endothelial cell surface is dramatically reduced and the levels of pro-adhesives molecules (such as ICAM) on the endothelium increase, resulting in neutrophil accumulation. The researchers showed both in tissue culture and animal models that the adherence and infiltration of inflammatory cells could be blocked by experimentally expressing excess endomucin in the vascular endothelium.
"Until now researchers studying the role of the endothelium in inflammation have primarily focused on pro-adhesive molecules that trap the white blood cells at the site of injury," said Pablo Argüeso, Ph.D. "We have now shown that there is also a mechanism to maintain an anti-adhesive surface on the vascular endothelium. Endomucin acts to prevent the inflammatory cells from adhering to blood vessels. The fact that endomucin decreases during inflammation suggests that this molecule may be as important in transforming the endothelial cell surface to a proinflammatory state as the elevation in adhesive molecules."
Most current treatments for inflammation involve targeting the activities of cytokines and inflammatory mediators, which have risks and limitations. This new knowledge may be used to develop treatments for inflammation by promoting the expression of endomucin to prevent the movement of inflammatory cells from the capillaries into inflamed tissues.
"In our experiments, we have shown that there is potential to interfere with inflammation by promoting the expression of endomucin," said Dr. Argüeso. "Many diseases have an inflammatory component, and by targeting this molecule, we believe we can reduce unnecessary inflammation."
More information: Alisar Zahr et al. Endomucin prevents leukocyte–endothelial cell adhesion and has a critical role under resting and inflammatory conditions, Nature Communications (2016). DOI: 10.1038/ncomms10363